Abstract
Background
The association between treatment-related lymphopenia in multiple sclerosis, drug efficacy and the risk of infections is not yet fully understood.
Objective
The objective of this study was to assess whether lymphopenia is associated with short-term treatment response and infection rate in a real-life multiple sclerosis population treated with fingolimod and dimethyl-fumarate. We assessed the associations between baseline absolute lymphocyte count and the lymphocyte mean percentage decrease at 6 and 12 months with treatment response and the occurrence of adverse events over 12 months in the entire cohort of patients and in the two treatment groups separately.
Methods
This is a retrospective observational real-world study of patients with multiple sclerosis treated with fingolimod and dimethyl-fumarate at the MS Center of the University of Genoa between 2011 and 2018. Patients with at least 12 months of follow-up were eligible if [1] they had an Expanded Disability Status Scale assessment at baseline and 12 months after treatment onset, [2] they had undergone brain magnetic resonance imaging at baseline and after 12 months, and [3] absolute lymphocyte counts were available at baseline, 6 and 12 months. Patients shifting from dimethyl-fumarate to fingolimod or vice versa were excluded from the analysis.
Results
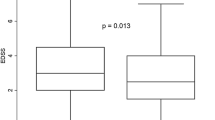
In total, 137 and 75 patients treated with fingolimod and dimethyl-fumarate, respectively, were included in the analysis. At 12 months, fingolimod-treated patients were more likely to experience grade II and grade III lymphopenia compared with dimethyl-fumarate patients (p < 0.001, χ2 = 94) and had a higher lymphocyte mean percentage decrease (p < 0.001, U = 540). A higher number of previous therapies and a lower baseline absolute lymphocyte count were predictors of lymphopenia at 6 months (p = 0.047, odds ratio = 1.60 and p = 0.014, odds ratio = 1.1) and 12 months (p = 0.003, odds ratio = 1.97 and p = 0.023, odds ratio = 1.1). In fingolimod-treated patients only, female sex and a higher Expanded Disability Status Scale score were predictors of lymphopenia at 12 months (p = 0.006, odds ratio = 7.58 and p = 0.03, odds ratio = 1.56). Neither absolute lymphocyte count at 6 and 12 months nor the mean percentage decrease at 6 and 12 months predicted No Evidence of Disease Activity (NEDA-3) status at 1 year, the occurrence of relapses, disease activity on MRI or disability progression.
Conclusions
Our findings suggest that peripheral blood lymphocyte changes are not associated with short-term treatment response and with the rate of infections during fingolimod and dimethyl-fumarate treatment in real-world patients. Higher treatment exposure and a lower baseline absolute lymphocyte count are risk factors for lymphopenia development during fingolimod and dimethyl-fumarate therapy.

Similar content being viewed by others
References
Fox EJ, Buckle GJ, Singer B, Singh V, Boster A. Lymphopenia and DMTs for relapsing forms of MS: considerations for the treating neurologist. Neurol Clin Pract. 2019;9(1):53–63.
Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Calabresi PA, Radue E-W, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–56.
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107.
Fragoso YD, Spelman T, Boz C, Alroughani R, Lugaresi A, Vucic S, et al. Lymphocyte count in peripheral blood is not associated with the level of clinical response to treatment with fingolimod. Mult Scler Relat Disord. 2018;19:105–8.
Longbrake EE, Naismith RT, Parks BJ, Wu GF, Cross AH. Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Mult Scler J Exp Transl Clin. 2015;1:205521731559699. https://doi.org/10.1177/2055217315596994.
Wright K, Winkler MD, Newton BD, Sormani MP, Okuda DT. Patient outcomes influenced by reduced lymphocyte counts after dimethyl fumarate initiation. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):1–5.
Manni A, Direnzo V, Iaffaldano A, Di Lecce V, Tortorella C, Zoccolella S, et al. Gender differences in safety issues during fingolimod therapy: evidence from a real-life relapsing multiple sclerosis cohort. Brain Behav. 2017;7(10):1–7.
Fox RJ, Chan A, Gold R, Phillips JT, Selman K. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS. Neurol Clin Pract. 2016;6(3):220–9. https://doi.org/10.1212/CPJ.0000000000000238.
Fox EJ, Lublin FD, Wolinsky JS, Cohen JA, Williams IM, Meng X, et al. Lymphocyte counts and infection rates. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e614. https://doi.org/10.1212/NXI.0000000000000614.
de la Maza SS, Medina S, Villarrubia N, Costa-Frossard L, Monreal E, Tejeda-Velarde A, et al. Factors associated with dimethyl fumarate-induced lymphopenia. J Neurol Sci. 2019;398:4–8. https://doi.org/10.1016/j.jns.2019.01.007.
Warnke C, Dehmel T, Ramanujam R, Holmen C, Nordin N, Wolfram K, et al. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology. 2014;83(23):2153–7.
Ohtani R, Mori M, Uchida T, Uzawa A, Masuda H, Liu J, et al. Risk factors for fingolimod-induced lymphopenia in multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4(1):205521731875969.
Baharnoori M, Gonzalez CT, Chua A, Diaz-Cruz C, Healy BC, Stankiewicz J, et al. Predictors of hematological abnormalities in multiple sclerosis patients treated with fingolimod and dimethyl fumarate and impact of treatment switch on lymphocyte and leukocyte count. Mult Scler Relat Disord. 2018;20:51–7.
Morales FS, Koralnik IJ, Gautam S, Samaan S, Sloane JA. Risk factors for lymphopenia in patients with relapsing-remitting multiple sclerosis treated with dimethyl fumarate. J Neurol. 2019. https://doi.org/10.1007/s00415-019-09557-w.
Paolicelli D, Manni A, D’Onghia M, Direnzo V, Iaffaldano P, Zoccolella S, et al. Lymphocyte subsets as biomarkers of therapeutic response in fingolimod treated relapsing multiple sclerosis patients. J Neuroimmunol. 2017;303:75–80. https://doi.org/10.1016/j.jneuroim.2016.12.012.
Quirant-Sánchez B, Hervás-García JV, Teniente-Serra A, Brieva L, Moral-Torres E, Cano A, et al. Predicting therapeutic response to fingolimod treatment in multiple sclerosis patients. CNS Neurosci Ther. 2018;24(12):1175–84. https://doi.org/10.1111/cns.12851.
Manni A, Iaffaldano A, Lucisano G, D’Onghia M, Mezzapesa DM, Felica V, et al. Lymphocyte count and body mass index as biomarkers of early treatment response in a multiple sclerosis dimethyl fumarate-treated cohort. Front Immunol. 2019;14(10):1343. https://doi.org/10.3389/fimmu.2019.01343/full.
Mansilla MJ, Navarro-Barriuso J, Presas-Rodríguez S, Teniente-Serra A, Quirant-Sánchez B, Ramo-Tello C, et al. Optimal response to dimethyl fumarate is mediated by a reduction of Th1-like Th17 cells after 3 months of treatment. CNS Neurosci Ther. 2019;25(9):995–1005. https://doi.org/10.1111/cns.13142.
Pagani F, Testi C, Grimaldi A, Corsi G, Cortese B, Basilico B, et al. Dimethyl fumarate reduces microglia functional response to tissue damage and favors brain iron homeostasis. Neuroscience [Internet]. 2019 Nov; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306452219307407.
Traub J, Traffehn S, Ochs J, Häusser-Kinzel S, Stephan S, Scannevin R, et al. Dimethyl fumarate impairs differentiated B cells and fosters central nervous system integrity in treatment of multiple sclerosis. Brain Pathol. 2019;29(5):640–57. https://doi.org/10.1111/bpa.12711.
Montes Diaz G, Hupperts R, Fraussen J, Somers V. Dimethyl fumarate treatment in multiple sclerosis: recent advances in clinical and immunological studies. Autoimmun Rev. 2018;17(12):1240–50. https://doi.org/10.1016/j.autrev.2018.07.001.
Kim S, Bielawski J, Yang H, Kong Y, Zhou B, Li J. Functional antagonism of sphingosine-1-phosphate receptor 1 prevents cuprizone-induced demyelination. Glia. 2018;66(3):654–69. https://doi.org/10.1002/glia.23272.
Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation. 2011;8(1):76. https://doi.org/10.1186/1742-2094-8-76.
Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, Caverzasi E, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–66. https://doi.org/10.1002/ana.25463.
Winkelmann A, Loebermann M, Reisinger EC, Hartung H-P, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217–33. https://doi.org/10.1038/nrneurol.2016.21.
Perini P, Rinaldi F, Puthenparampil M, Marcon M, Perini F, Gallo P. Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient. Mult Scler Relat Disord. 2018;26:68–70. https://doi.org/10.1016/j.msard.2018.09.009.
Greenstein JI. Diffuse dermatophytosis occurring on dimethyl fumarate therapy. Mult Scler J. 2018;24(7):999–1001. https://doi.org/10.1177/1352458517741207.
Mehta D, Miller C, Arnold DL, Bame E, Bar-Or A, Gold R, et al. Effect of dimethyl fumarate on lymphocytes in RRMS: implications for clinical practice. Neurology. 2019;92(15):e1724–38.
Kaufmann M, Haase R, Proschmann U, Ziemssen T, Akgün K. Real world lab data: patterns of lymphocyte counts in fingolimod treated patients. Front Immunol. 2018;9:1–11.
Nakhaei-Nejad M, Barilla D, Lee CH, Blevins G, Giuliani F. Characterization of lymphopenia in patients with MS treated with dimethyl fumarate and fingolimod. Neurol Neuroimmunol Neuroinflamm. 2018;5(2):1–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the conduct of this study or the preparation of this article.
Conflict of Interest
Giacomo Boffa, Nicolò Bruschi, Maria Cellerino, Giovanni Novi, Elvira Sbragia and Elisabetta Capello have no conflicts of interest that are directly relevant to the content of this study. Caterina Lapucci received honoraria for travel expenses for attending meetings from Genzyme and Roche. Antonio Uccelli received grants and contracts from the Italian Multiple Sclerosis Society Foundation (FISM), Novartis, Fondazione Cariplo, and the Italian Ministry of Health and received honoraria or consultation fees from Biogen, Roche, Teva, Merck, Genzyme and Novartis. Matilde Inglese received grants from the National Institutes of Health, National Multiple Sclerosis Society and FISM and received fees for consultation from Roche, Genzyme, Merck, Biogen and Novartis.
Ethics Approval
This study was approved by the local ethical committee (Comitato Etico Regionale Liguria, San Martino).
Consent to Participate
All patients signed a written informed consent before the study.
Rights and permissions
About this article
Cite this article
Boffa, G., Bruschi, N., Cellerino, M. et al. Fingolimod and Dimethyl-Fumarate-Derived Lymphopenia is not Associated with Short-Term Treatment Response and Risk of Infections in a Real-Life MS Population. CNS Drugs 34, 425–432 (2020). https://doi.org/10.1007/s40263-020-00714-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00714-8