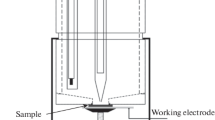
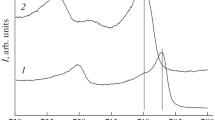
Abstract—
The results of a study of the morphology and chemical structure of iron coatings deposited on nanostructured surfaces of aluminum and porous alumina by the thermal deposition method before and after the cathodic hydrogen evolution reaction are reported. It is shown that an increase in the diameter of pores on the surface of alumina does not lead to a change in the cathode current density due to the different contribution of the boundary between the pores to the total surface area of the sample, whereas an increase in the size of hemispherical pits on the surface of aluminum plates leads to an increase in the cathode current density, which indicates an increase in the hydrogen evolution efficiency. No changes are detected in the morphology and chemical structure of the surface before or after the reaction.







Similar content being viewed by others
REFERENCES
L. A. Kibler, Chem. Phys. Chem. 7, 985 (2006). https://doi.org/10.1002/cPhc.200500646
M. R. Gennero de Chialvo and A. C. Chialvo, Phys. Chem. Chem. Phys. 3, 3180 (2001). https://doi.org/10.1039/B102777H
J. O. Gil Posada, and P. J. Hall, Int. J. Hydrogen Energy 41, 20807 (2016). https://doi.org/10.1016/j.ijhydene.2016.04.123
Y. Attia and M. Samer, Renewable Sustainable Energy Rev. 79, 878 (2017). https://doi.org/10.1016/j.rser.2017.05.113
Y. J. Wang, S. M. Hussain, and G. P. Krestin, Eur. Radiol. 11, 2319 (2001). https://doi.org/10.1007/s003300100908
A. Jordan, R. Scholz, P. Wust, et al., J. Magn. Magn. Mater. 201, 413 (1999). https://doi.org/10.1016/S0304-8853(99)00088-8
C. G. Hadji Panayis, M. J. Bonder, S. Balakrishnan, et al., Small 4, 1925 (2008). https://doi.org/10.1002/smll.200800261
X. Li, D. W. Elliott, and W. Zhang, Crit. Rev. Solid State Mater. Sci. 31, 111 (2006). https://doi.org/10.1080/10408430601057611
W. Yan, H. L. Lien, B. E. Koel, and W. Zhang, Environ. Sci. Process. Impacts 15, 63 (2013). https://doi.org/10.1039/C2EM30691C
G. S. Nechitailo, O. A. Bogoslovskaya, I. P. Ol’khovskay, and N. N. Glushchenko, Nanotechnol. Russ. 13, 161 (2018). https://doi.org/10.1134/S1995078018020052
H. Föll, M. Christophersen, J. Carstensen, and G. Hasse, Mater. Sci. Eng. R. 39, 93 (2002). https://doi.org/10.1016/s0927-796x(02)00090-6
R. G. Valeev, A. L. Trigub, A. N. Beltiukov, et al., J. Surf. Invest.: X-ray, Synchrotr. Neutron Tech. 13, 92 (2019). https://doi.org/10.1134/S1027451019010373
R. G. Valeev, V. V. Stashkova, A. I. Chukavin, et al., Phys. Proc. C 84, 407 (2016). https://doi.org/10.1016/j.PhPro.2016.11.069
R. S. Patil, C. D. Lokhe, and R. S. Mane, et al., J. Non-Cryst. Solids 353, 1645 (2007). https://doi.org/10.1016/j.jnoncrysol.2007.01.014
L. Messel and R. Glang, Handbook of Thin Film Technology (McGraw-Hill, New York, 1970), Vol. 2.
H. Masuda and K. Fukuda, Science (Washington, DC, U. S.) 268, 1466 (1995). https://doi.org/10.1126/science.268.5216.1466
A. Santos, P. Formentin, J. Pallares, et al., J. Electroanal. Chem. 655, 73 (2011). https://doi.org/10.1016/j.jelechem.2011.02.005
C. A. Schneider, W. S. Rasband, and K. W. Eliceiri, Nat. Methods 9, 671 (2012). https://doi.org/10.1038/nmeth.2089
Y. Zheng, Y. Jiao, M. Jaroniec, and S. Z. Qiao, Angew. Chem., Int. Ed. 54, 52 (2015). https://doi.org/10.1002/anie.201407031
N. Eliaz and E. Gileadi, Physical Electrochemistry: Fundamentals, Techniques, and Applications, 2nd ed. (Wiley, New York, 2019).
Funding
This study was conducted using the equipment of the Center for Collective Use, Center of Physical and Physicochemical Methods of Analysis, Investigation of Properties and Characteristics of the Surface, Nanostructures, Materials, and Products, Udmurt Federal Research Center, Ural Branch, Russian Academy of Sciences, with financial support from the Ministry of Science and Higher Education of the Russian Federation within the framework of Federal Target Program Research and Development in Priority Areas of Development of the Russian Scientific and Technological Complex for 2014–2020 (unique identification code of the project RFMEFI62119X0035) within the fundamental research project of the Ural Branch of the Russian Academy of Sciences (project no. 18-10-2-25), as well as within the topic of the Department of Surface Physics and Chemistry, Udmurt Federal Research Center, Ural Branch, Russian Academy of Sciences (state registration number AAAA-A17-117022250040-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Valeev, R.G., Alalykin, A.S. Morphology, Chemical Structure, and Cathode Properties of Nanostructured Iron Coatings on Highly Developed Surfaces of Aluminum and Porous Alumina. Nanotechnol Russia 14, 346–352 (2019). https://doi.org/10.1134/S199507801904013X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199507801904013X