Abstract
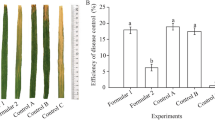
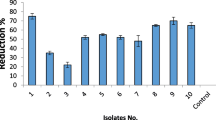
The aim of the present study was to focus on the growth promotion and wilt diseases protection of tomato plants by Bacillus sp. LBF-01.The plant growth promotion (both in vitro and in vivo) and diseases protection mechanism were studied following standard protocols. This bacterial strain showed positive PGP and antifungal traits against the wilt pathogen Fusarium sp. The seed bacterization with Bacillus sp. LBF-01 improved the germination rate of tomato seeds by 20% compared to the control whereas the vigor index was increased by 854.026%.The percentage of disease protection by Bacillus sp. LBF-01 against root-rot disease inoculated was inversely proportional to the doses of fungal spores inoculum. Under in vivo condition, the PGP bacteria enhanced the seedling growth in nursery as well as field condition. Application of Bacillus sp. LBF-01 enhanced the activity of defense related enzymes in root and leaves. Bacillus sp. LBF-01 treatment had significantly enhanced total chlorophyll and carotenoids contents of leaves by 77.78 and 52.5% over untreated control plants. It is evident from the present study that the application of the Bacillus sp. LBF-01 bacterial strain in tomato soil improved the potentiality of the plant by systemic resistance and control the wilt diseases for tomato production.






Similar content being viewed by others
REFERENCES
McGovern, R.J., Management of tomato diseases caused by Fusarium oxysporum,Crop Prot., 2015, no. 73, pp. 78–92. https://doi.org/10.1016/j.cropro.2015.02.0210261-2194
Lim, J.H., Kim, M.S., Kim, H.E., Yano, T., Oshima, Y., Agarwal, K., Goldman, W.E., Silverman, N., Kurata, S., and Oh, B.-Ha, Structural basis for preferential recognition of diaminopimelic acid-type peptidoglycan by a subset of peptidoglycan recognition proteins, J. Biol. Chem., 2006, vol. 281, no. 12, pp. 8286–8295.
Castaño, R., Borrero, O.C., Trillas, M.I., and Avilés, M., Selection of biological control agents against tomato Fusarium wilt and evaluation in greenhouse conditions of two selected agents in three growing media, BioControl, 2013, no. 58, pp. 105–116. https://doi.org/10.1007/s10526-012-9465-z
Vethavalli, S. and Sudha, S.S., In vitro and in silico studies on biocontrol agent of bacterial strains against Fusarium oxysporum f. sp. lycopersici, Res. Biotechnol., 2012, no. 3, pp. 22–31.
Ying Cao, Xiaofeng Tang, Jim Giovannoni, Fangming Xiao, and Yongsheng Liu, Functional characterization of a tomato COBRA-like gene functioning in fruit development and ripening, BMC Plant Biol., 2012, vol. 12. https://doi.org/10.1186/1471-2229-12-211
Baysal, Ö., Çalışkan, M., and Yeşilova, Ö., An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici, Physiol. Mol. Plant. Pathol., 2008, vol. 73, nos. 1–3, pp. 25–32. https://doi.org/10.1016/j.pmpp.2008.11.002
Hartmann, A., Schmid, M., van Tuinen, D., and Berg, G., Plant-driven selection of microbes, Plant Soil, 2009, no. 321, pp. 235–257. https://doi.org/10.1007/s11104-008-9814-y
Kloepper, J.W., Ryu, C.M., and Zhang, S., Induced systemic resistance and promotion of plant growth by Bacillus spp., Phytopathology, 2004, no. 94, pp. 1259–1266. https://doi.org/10.1094/PHYTO.2004.94.11.1259
Ongena, M.I. and Jacques, P., Bacillus lipopeptides: Versatile weapons for plant disease biocontrol, Trends Microbiol., 2008, vol. 16, no. 3, pp. 115–25. https://doi.org/10.1016/j.tim.2007.12.009
Abdallah, R.A.B., Stedel, C., Garagounis, C., Nefzi, A., Jabnoun-Khiareddine, H., Papadopoulou, K.K., and Daami-Remadi, M., Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato, Crop Prot., 2017, no. 99, pp. 45–58. https://doi.org/10.1016/j.cropro.2017.05.008
El-Sayed, W.S., Akhkha, A., El-Naggar, M.Y., and Elbadry, M., In vitro antagonistic activity, plant growth promotingtraits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil, Front Microbiol., 2014, vol. 5, p. 651. https://doi.org/10.3389/fmicb.2014.00651
Schwyn, B. and Neilands, J., Universal chemical assay for the detection and determination of siderophores, Anal. Biochem., 1987, vol. 160, no. 1, pp. 47–56. https://www.ncbi.nlm.nih.gov/pubmed/2952030.
Shaw, F.J., Lin, P.F., Chen, C.S., and Chen, C.H., Purification and properties of an extracellular α-amylase from Thermus sp., Bot. Bull. Acad. Sin., 1995, no. 36, pp. 195–200. https://ejournal.sinica.edu.tw/bbas/content/1995/3/bot363-08.pdf.
Nautiyal, C.S., An efficient microbiological growth medium for screening phosphate solubilizing microorganisms, FEMS Microbiol. Lett., 1999, vol. 170, no. 1, pp. 265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Robert, W.K. and Selitrennikoff, C.P., Plant and bacterial chitinase differ in antifungal activity, J. Gen. Microbiol., 1988, no. 134, pp. 169–176. https://doi.org/10.1099/00221287-134-1-169
Walsh, G.A., Murphy, R.A., Killeen, G.F., Headon, D.R., and Power, R.F., Technical note: Detection and quantification of supplemental fungal β-glucanase activity in animal feed, J. Anim. Sci., 1995, vol. 73, no. 4, pp. 1074–1076. https://www.ncbi.nlm.nih.gov/pubmed/7628950.
Gordon, S.A. and Weber, R.P., Colorimetric estimation of indole-acetic acid, Plant Physiol., 1951, no. 26, pp. 192–195.
Cappuccino, J.C. and Sherman, N., Negative staining, in Microbiology: A Laboratory Manual, Cappuccino, J.C. and Sherman, N., Ed., Benjamin/Cummings Redwood City, 1992, pp. 125–179.
Nautiyal, C.S., An efficient microbiological growth medium for screening phosphate solubilizing microorganisms, FEMS Microbiol. Lett., 1999, vol. 170, no. 1, pp. 265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Hossain, M.M., Sultana, F., Kubota, M., and Hyakumachi, M., Differential inducible defense mechanisms against bacterial speck pathogen in Arabidopsis thaliana by plant-growth-promoting-fungus Penicillium sp. GP16-2 and its cell free filtrate, Plant Soil, 2008, no. 304, pp. 227–239. https://doi.org/10.1007/s11104-008-9542-3
Deora, A., Hashidoko, Y., Islam, M.T., and Tahara, S., Antagonistic rhizoplane bacteria induce diverse morphological alterations in Peronosporomycete hyphae during in vitro interaction, Eur. J. Plant Pathol., 2005, no. 112, pp. 311–322. https://doi.org/10.1007/s10658-005-4753-4
Khan, A.L., Waqas, M., Hussain, J., Al-Harrasi, A., Al-Rawahi, A., and Al-Hosni, K., Endophytes Aspergillus caespitosus LK12 and Phoma sp. LK13 of Moringa peregrina produce gibberellins and improve rice plant growth, J. Plant. Interact., 2014, no. 9, pp. 731–737. https://doi.org/10.1080/17429145.2014.917384
Montealegre, J.R., Reyes, R., Perez, L.M., Herrera, R., Silva, P., and Besoain, X., Selection of bioantagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato, Electron. J. Biotechnol., 2003, no. 6, pp. 115–127.
Naureen, Z., Price, A.H., Hafeez, F.Y., and Roberts, M.R., Identification of rice blast disease suppressing bacterial strains from the rhizosphere of rice grown in Pakistan, Crop Prot., 2009, no. 28, pp. 1052–1060.
Ge, B., Liu, B., Nwet, T.T., Zhao, W., Shi, L., and Zhang, K., Bacillus methylotrophicus strain NKG-1, isolated from Changbai Mountain, China, has potential applications as a biofertilizer or biocontrol agent, PLoS ONE, 2016, no. 11, pp. 1–13, e0166079. https://doi.org/10.1371/journal.pone.0166079
Abeles, B., Bosshart, P., Forrence, L.E., and Habiz, W., Preparation and purification of glucanase and chitinase frombean leaves, Plant Physiol., 1970, no. 1, pp. 129–134.
Lichtenthaler, H.K., Chlorophylls and carotenoids: Pigments of photosynthetic membranes, Method Enzymol., 1987, no. 148, pp. 350–382.
ACKNOWLEDGMENTS
The authors would like to thank Projesh Dutta, Guest Lecturer of Gour Mahavidyalaya, Malda for Statistical analysis. The authors are also grateful to Sohini Chakraborty, Research Scholar, Department of Botany, University of Gour Banga, for estimation of defense enzyme and photosynthetic pigments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Swapan Kumar Chowdhury, Majumdar, S. & Mandal, V. Biocontrol Potential and Growth Promotion Capability of Bacillus sp. LBF-1 for Management of Wilt Disease of Solanum lycopersicum Caused by Fusarium sp.. Russ. Agricult. Sci. 46, 139–147 (2020). https://doi.org/10.3103/S1068367420020044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367420020044