Abstract
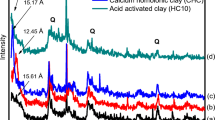
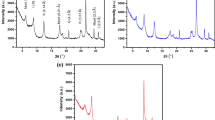
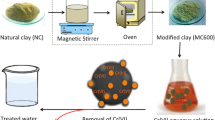
Copper is toxic and is considered as the hazardous pollutant due to his stability in the environment. Current technologies used for its removal involve materials which can be difficult to synthesize, are expensive or are themselves potentially toxic. Natural clays are abundant worldwide, relatively cheap, possess sorption and ion exchange properties, are candidates as adsorbents. While the Cu(II) sorption capacity of raw bentonite is relatively low, modified bentonites represent a new class of sorbents for effective Cu(II) removal from wastewater. The present study investigates the influence of Algerian clay modification on the capacity of copper removal from water. This montmorillonite, which is a clay mineral of the smectite group, possesses silica tetrahedral sheets layered between alumina octahedral sheets. Several adsorbents were prepared from this bentonite by saturation with sodium, calcium and treatment with sulphuric acid to produce three adsorbents, ARS, ARC and ARH, respectively. The three materials obtained were tested for the Cu(II) adsorption from aqueous solutions. The adsorbents and metal interactions were studied under different conditions of interaction time, pH, concentration of metal ions and amount of clay. It was found that the interactions were dependent on pH, the uptake of pollutant was controlled by the amount of clay and the initial copper concentration. Langmuir and Freundlich models were fitted to experimental isotherms. The Langmuir model shows a better fit to the Cu ions adsorption isotherm for all systems. The largest adsorption capacity is observed for sodium homoionic clay. The Langmuir maximum sorption capacity of Cu(II) ions on ARH, ARC and ARS was found to be 17.241, 18.181 and 24.390 mg/g, respectively. The three adsorbents also showed a high efficiency in the Cu(II) adsorption from much diluted solutions. This work suggested that the modified clays can be promising candidates for the removal of copper ions from aqueous solutions.
Similar content being viewed by others
References
Sfakianakis, D.G, Renieri, E., Kentouri, M., and Tsatsakis, A.M., Effect of heavy metals on fish larvae deformities: A review, Environ. Res., 2015, vol. 137, pp. 246–255.
Chowdhury, S., Mazumder, M.A.J.; Al-Attas, O., and Husai, T., Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries, Sci. Total Environ., 2016, vol. 569/570, pp. 476–488.
Varun, M., D’Souza, R., Pratas, J., and Paul, M.S., Metal contamination of soils and plants associated with the glass industry in North Central India: prospects of phytoremediation, Environ. Sci. Pollut. Res., 2012, vol. 19, pp. 269–281.
Naser, H.A., Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: A review, Mar. Pollut. Bull., 2013, vol. 72, no. 1, pp. 6–13.
Alyemeni, M.N and Almohisen, I.A.A., Traffic and industrial activities around Riyadh cause the accumulation of heavy metals in legumes: A case study, Saudi J. Biol. Sci., 2014, vol. 21, pp. 167–172.
Wang, S. and Mulligan, C.N., Effect of natural organic matter on arsenic release from soils and sediments into groundwater, Environ. Geochem. Health, 2006, vol. 28, pp. 197–214.
Oucher, N., Kerbachi, R. Ghezloun, A., and Merabet, H., Magnitude of Air Pollution by Heavy Metals Associated with Aerosols Particles in Algiers, Energy Procedia, 2015, vol. 74, pp. 51–58.
Cegowskaa, A., Sokoowska, K., Samecka-Cymermana, A., Kolona, K., Jusikc, S., and Kempers, A.J., Copper and zinc in Elodea Canadensis from rivers with various pollution levels, Ecol. Indic, 2016, vol. 67, pp. 156–165.
Bitencourt, J.A.P, Pereira, D.C., da Silva Neto, I.D, and Crapez, M.A.C., The toxic effect of copper on the association between ciliates Euplotes vannus and Euplotes crassus and their naturally associated bacteria isolated from a polluted tropical bay, Reg. Stud. Mar. Sci., 2016, vol. 3, pp. 25–32.
Gündogan, R., Acemioglu, B., and Alma, M.H., Copper (II) adsorption from aqueous solution by herbaceous peat, Colloid Interface Sci, 2004, vol. 269, pp. 303–309.
Kavak, D., Removal of lead from aqueous solutions by precipitation: statistical analysis and modeling, Desal. Water Treat., 2013, vol. 51, pp. 1720–1726.
Fersi Bennani, C. and M’hiri, O., Comparative study of the removal of heavy metals by two nanofiltration membranes, Desal. Water Treat., 2015, vol. 53, pp. 1024–1030.
Dialynas, E. and Diamadopoulos, E., Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater, Desalination, 2009, vol. 238, no. 1–3, pp. 302–311.
Chen, S., Chen, W, and Shih, C., Heavy metal removal from wastewater using zero-valent iron nanoparticles, Water Sci. Technol., 2008, vol. 58, no. 10, pp. 1947–1954.
Yuan, X.Z., Meng, Y.T., Zeng, G.M., Fang, Y.Y., and Shi, J.G., Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation, Colloids Surf., A, 2008, vol. 317, pp. 256–261.
Hunsom, M., Pruksathorn, K., Damronglerd, S., Vergnes, H., and Duverneuil, P., Electrochemical treatment of heavymetals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction, Water Res., 2005, vol. 39, pp. 610–616.
Malkoc, E., Nuhoglu, Y., and Dundar, M., Adsorption of chromium (VI) on pomace-An olive oil industry waste: Batch and column studies, J. Hazard. Mater., 2016, vol. 138, pp. 142–151.
Ray, P.Z. and Shipley, H.J., Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review, RSC Adv., 2015, vol. 5, pp. 29885–29907.
Goncharuk, V.V., Puzyrnaya, L.N., Pshinko, G.N., Kosorukov, A.A., and Demchenko, V.Ya., Removal of Cu (II), Ni (II), and Co (II) from aqueous solutions using layered double hydroxide intercalated with EDTA, J. Water Chem. and Technol., 2011, vol. 33, no. 5, pp. 488–495.
Ma, L., Chen, Q., Zhu, J., Xi, Y., He, H., Zhu, R., Tao, Q., and Ayoko, G.A., Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems, Chem. Eng. J., 2016, vol. 283, pp. 880–888.
Zhi-rong, L. and Shao-qi, Z., Adsorption of copper and nickel on Na-bentonite, Process Saf. Environ. Prot., 2010, vol. 88, pp. 62–66.
Pawar, R.R., Lalhmunsiama Bajaj, H.C., and Lee, S.-M., Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: Batch and column studies, J. Ind. Eng. Chem., 2016, vol. 34, pp. 213–223.
Soleimani. M. and Siahpoosh. Z.H., Ghezeljeh nanoclay as a new natural adsorbent for the removal of copper and mercury ions: Equilibrium, kinetics and thermodynamics studies, Chinese J. Chem. Eng., 2015, vol. 23, no. 11, pp. 819–1833.
Belaroui, L.S., Millet, J.M.M., and Bengueddach, A., Characterization of lalithe, a new bentonite-type Algerian clay, for intercalation and catalysts preparation, Catal. Today, 2004, vol. 89, pp. 279–286.
Li, S.-Z. and Wu, P.-X., Characterization of sodium dodecyl sulfate modified iron pillared montmorillonite and its application for the removal of aqueous Cu (II) and Co (II), J. Hazard. Mater., 2010, vol. 173, pp. 62–70.
Yu, B., Zhang, Y., Shukla, A., Shukla, S.S., and Dorris, K.L., The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper, J. Hazard. Mater., 2000, vol. 80, pp. 33–42.
Yang, S., Zhao, D., Zhang, H., Lu, S., Chen, L., and Yu, X., Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions, J. Hazard. Mater., 2010, vol. 183, pp. 632–640.
Jain, C.K. and Ram, D., Adsorption of lead and zinc on bed sediments of the river kali, Water Res., 1997, vol. 31, pp. 154–162.
Abollino, O., Giacomino, A., Malandrino, M., and Mentasti, E., Interaction of metal ions with montmorillonite and vermiculite, Appl. Clay Sci., 2008, vol. 38, pp. 227–236.
Liu, X., Hicher, P., Muresan, B., Saiyouri, N., and Hicher, P.-Y., Heavy metal retention properties of kaolin and bentonite in a wide range of concentration and different pH conditions, Appl. Clay Sci., 2016, vol. 119, pp. 365–374.
Langmuir, I., The adsorption of gases on plane surfaces of glass, mica and platinum, J. Chem. Soc., 1918, vol. 40, pp. 1361–1403.
Freundlich, H.M.F., Über die adsorption in lösungen, Z. Phys. Chem., 1906, vol. 57, S. 385–470.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
About this article
Cite this article
Belhadri, M., Sassi, M. & Bengueddach, A. Preparation of Economical and Environmentaly Friendly Modified Clay and Its Application for Copper Removal. J. Water Chem. Technol. 41, 357–362 (2019). https://doi.org/10.3103/S1063455X19060031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X19060031