Abstract
The NOx in the exhaust gas emitted from the coke combustion and sintering process has a serious impact on the environment. How to reduce the release of NOx in the combustion of coke has become a major problem to be solved. Adding a certain amount of catalyst to the coke combustion process can not only effectively improve the combustion efficiency of coke but also reduce the NOx emissions. In this paper, CeO2 is used as an inhibitor for coke combustion experiments, micro-sintering experiments and sintering cup experiments, focusing on the effect of CeO2 on NOx emissions during the coke combustion and sintering process. It is found that in the coke combustion reaction the addition of 1 and 2% CeO2 resulted in NOx reduction of 5.6 and 2.5%. The addition of 3% CeO2 resulted in a 2.8% increase in NOx emissions. When the amount of CeO2 added was 2%, the NO reduction rate during the sintering cup test reached 25.3%. At the same time, the addition of 2% CeO2 resulted in little change in the yield of the sinter, and the increase of the drum strength of the sinter.
Similar content being viewed by others
INTRODUCTION
Nitrogen oxides are one of the main air pollutants, which are the main cause of acid rain, photochemical smog and haze. The sinter plant in China’s steel companies is a source of pollution that produces more NOx [1, 2]. Reducing the problem of nitrogen oxides emission during sintering Process is a century-long problem in the steel industry. The NOx emitted by these sinter plants is mainly derived from the NOx emissions from coke combustion. And emission reduction has become a major problem that needs to be solved urgently [3, 4].
Nowadays, there are mainly the following denitration technologies at home and abroad: flue gas cycle denitration technology, selective non-catalytic reduction (SNCR) denitration technology and selective catalytic reduction (SCR) denitration technology [5–7]. Due to various conditions of the above denitration technology, experts and scholars at home and abroad are paying more and more attention to the development of inhibitors that can effectively reduce NOx emissions during combustion process. The denitrification inhibitors mainly convert nitrogen from NOx into N2, thereby reducing NOx emissions. The development of denitrification inhibitors not only solves the problem of insufficient nitrogen content of coal or coke reserves, but also saves the cost of flue gas denitration equipment. Therefore, this technology has gradually attracted the attention of experts and scholars.
Yanguang Chen et al. [8–11] invented a new sintering process. The sintering experiment was carried out by adding the modified coke powder under different process conditions in a certain proportion in the sintering material. The results showed that the modified coke is indeed possible to reduce the amount of NOx released during the sintering process. The addition of K2O, CaO and CeO2 to coke can catalyze the conversion of NOx to N2, thus reducing NOx emissions, and the three denitrification capacities are ranked as CeO2, CaO, K2O from large to small. In the micro-sintering experiment, when the coke powder modified with 2.0% cerium oxide and 2.0% calcium oxide was used as fuel in the sinter, the NOx removal rate was found to be 18.8 and 13.5%, respectively. However, the effect of the addition of inhibitors on the quality and yield of sinter has not been reported. In this paper, CeO2 is used as an inhibitor to carry out coke combustion experiments, micro-sintering experiments and sintering cup experiments, focusing on the effect of CeO2 on NOx emissions during the coke combustion and sintering process.
EXPERIMENTAL
The experimental raw materials were coke, quicklime, and Sintering material obtained from Meishan Iron and Steel Co., Ltd.
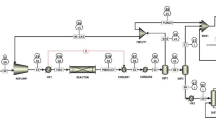
Experimental apparatus is shown in Fig. 1. The horizontal tube furnace system is mainly composed of air pump, furnace, flow controller and flue gas analyzer. In the experiment, the coke sample was loaded into a small boat and pushed by the sample feeding device to the middle of the horizontal tube furnace; the heating rate of the furnace was 15°C/min, and the temperature was raised to the set temperature to maintain the constant temperature. The flue gas generated during the combustion is analyzed by the British Kane KM9106 gas analyzer to analyze the concentration of each component in the flue gas, collect and record data. Until the concentration of NOx drops to a very low level, the combustion is then stopped.
In the micro-sintering experiment, a stainless steel cup containing 250 g of sintering materials was placed in a constant temperature zone of a tube furnace that had been heated to 1100°C. Then the sintering experiment was carried out. The discharge of NO during the experiment is calculated as shown in (1) and (2):


In formula (1), m is the amount of NO produced in units of mg; Q is the gas flow rate, the unit is L/min; t1 and t2 are the start and end times, in min; CNO is the concentration of NO as a function of time, in mg/m3; t is the time, the unit is min.
In formula (2), the CNO unit is ppm; M is the molar mass of the NO molecule, and the unit is g/mol, and the others are the same as formula (1).
The emission reduction of NO during the experiment is calculated as shown in (3):
As in Eq. (3), V is the amount of NO emitted during the sintering process under different conditions, mg;
Vparent is NO emissions during sintering without the addition of inhibitors, mg.
RESULTS AND DISCUSSION
Effect of CeO2 Addition on the NOx Release during Coke Combustion
In this experiment, the effect of amount of CeO2 on the NOx emission during coke combustion was studied. The coke combustion experiment was carried out in a tube furnace. The concentration of NO and NO2 (mg/m3) in the flue gas discharged from the tail pipe was measured by a flue gas analyzer. The additive CeO2 is added to the coke by mechanical mixing. To ensure uniform mixing, sufficient mechanical stirring time is ensured each time.
As shown in Figs. 2 and 3, during coke combustion without addition of CeO2, the amount of NO released is significantly greater than the amount of NO2 released. The release of NO has three main peaks, which suggests that there may be three different types of nitrogen in coke. After the addition of CeO2 to coke, the peak of NO release changed due to the catalytic action of CeO2. It can be seen that the addition of 1–2% CeO2 leads to a significant decrease in NO release in the 40–50 min interval. As the amount of CeO2 added increased, the NO release peak height increased stepwise, indicating that the amount of CeO2 added significantly affected the release of NO. The release of NO2 is mainly concentrated between 430 and 630°C, and the addition of 1% CeO2 can obviously reduce the release of NO2. Conversely, more than 1% of CeO2 addition increased the release of NO2.
In order to study the performance of inhibitor addition to NO emission reduction, the NO emission reduction during the coke combustion process before and after the addition of inhibitors was studied. The results are shown in Fig. 4. As can be seen from Fig. 4, 1 and 2% CeO2 addition can reduce NO by 5.6 and 2.5%, respectively, during coke combustion compared to the absence of inhibitor addition. 3% of CeO2 addition resulted in an increase in NO release of 2.75%. The above results clearly show that the addition of CeO2 significantly affects the release of NO during coke combustion, and the addition of 1–2% CeO2 can reduce the release of NO. In the coke combustion process, the reaction of C–CO and CO–NO can reduce NO to N2. CeO2 can reduce the NO effect of the combustion process from the promotion of these two reactions by CeO2. When CeO2 is added in a large amount, it significantly promotes the formation of more NO between N2 and oxygen.
Effect of CeO2 Addition on NOx Release during Micro-Sintering
Figures 6 and 7 show the release curves of CO, NO and NO2 during micro-sintering. It can be seen that during the micro-sintering process, the concentrations of CO, NO and NO2 can be up to 1000, 800, and 50 mg/m3, respectively. The release range of CO, NO and NO2 is basically within 30 min and is released at the same time. After the addition of 0.5% CeO2 addition, the highest release concentrations of CO, NO and NO2 were significantly reduced from 1081, 804, 59 mg/m3 to 1050, 690 and 20 mg/m3, respectively. After the addition of 1% CeO2 addition, the maximum release concentrations of CO, NO and NO2 were reduced to 700, 500 and 17 mg/m3, respectively. After the addition of 2% CeO2 addition, the highest release concentration of CO increased, and the maximum release concentration of NO decreased little. As the amount of CeO2 continued to increase, the maximum release concentrations of CO and NO increased, indicating that 3% of CeO2 addition promoted NO release.
Figure 7 shows the effect of the amount of CeO2 added on NO reduction during micro-sintering. It can be seen from Fig. 7 that the addition of different amounts of CeO2 has different effects on the NOx emission during micro-sintering. The addition of 0.5, 1 and 2% CeO2 inhibits the release of NOx during the micro-sintering process. When the amount of CeO2 added is 0.5, 1 and 2%, the emission reduction of NO are about 14, 30 and 6.6%, respectively. When the amount of CeO2 added was 3%, the release of NO increased by 5.4%, which indicates that addition of 3% CeO2 promotes the conversion of nitrogenous compounds in the fuel coke to NO during the micro-sintering process. The above results are consistent with the effect of inhibitor addition on the release of NO during coke combustion. Figure 5 also shows that while NO is being reduced, CO is also reduced, indicating that the addition of CeO2 promotes the reaction between CO–NO. Therefore, the development of efficient NO reduction catalysts depends on whether they can promote the reaction between CO–NO.
Effect of CeO2 on NOx Release during Sintering Cup Experiment
Nitrogen in 5% coke used as a fuel during sintering is the main source of NO release during sintering. In order to verify whether CeO2 addition can reduce NO release during sintering, a 100 kg sintering cup experiment was performed. The sinter cup used in this experiment was supplied by Meisteel Co., Ltd., and the composition of the sintering materials is consistent with the composition of the sinter plant. The test was carried out on a 100 kg sintering experimental unit of Mei steel.
Figures 8–12 shows the release profiles of NO, NO2 and SO2 during sintering. It can be seen from Fig. 8 to Fig. 12 that the emission of NO basically starts to rise rapidly after ignition, rises to a higher level within 2 to 3 min, falls after the first peak, and then rises to 400 mg/m3 horizontal release platform in 200–600 s and finally the process of rapid decline after sintering is completed. It can be seen that there are two peaks in the release of SO2 without CeO2 addition. The first release peak of SO2 release after CeO2 addition disappears, which indicates that the addition of CeO2 inhibits the release of SO2. It can be seen from Fig. 12 that the addition of CeO2 can not only reduce the release level of NO during sintering but also shorten the release time of NO.
Figure 13 shows that the addition of CeO2 during the sintering process can significantly reduce the emissions of NO, NO2 and SO2 during the 100 kg sintering cup test. When the amount of CeO2 added was 2% (relative to the amount of coke in the sintering materials), the reduction rate of NO, NO2, and SO2 during the sintering process were 25.3, 97.2, and 25.5%, respectively. When the amount of CeO2 added was 3% (relative to the amount of coke in the sinter), the emission reductions of NO, NO2 and SO2 during the sintering process were 1.5, 50.6 and 34.6%, respectively. When the amount of CeO2 added was 4% (relative to the amount of coke in the sinter), the emission reductions of NO, NO2, and SO2 during the sintering process were 8.5, 93.1, and 19.8%, respectively. The above results show that the larger the amount of CeO2 added, the worse the effect of NO emission reduction, and the reduction effect of NO2 and SO2 does not change much. From the sintering time point of view, the addition of the inhibitor reduced the sintering time. When the CeO2 addition amount was 2 and 4%, respectively, the sintering time was shortened by 9 and 15%, respectively, which indicates that the addition of CeO2 can improve the sintering production efficiency.
Table 1 shows the effect of CeO2 addition on sintering drum strength and yield of sintered ore. It can be seen that the drum strength of the sinter increased with the addition of 2% CeO2, and the yield did not change much; the drum strength of the sinter was slightly weakened with the addition of 3% CeO2, and the yield change was small.
Mechanism of NO Conversion Catalyzed by CeO2
A large number of literatures have shown that the mechanism of removal of NOx by metal oxide in sintering flue gas is complicated. There are two main reactions, one is homogeneous reduction reaction (the reaction between CO and NO) and the heterogeneous reduction reaction refers to the reduction reaction between coke and NO. Both Figs. 5, 6 and Figs. 8–11 show that the decrease in NO concentration is accompanied by a decrease in CO concentration during coal combustion and during sintering, indicating that the addition of CeO2 promotes the reaction between CO and NO. The reaction between C and NO occurs simultaneously in the coke combustion process. The addition of CeO2 promotes coke combustion to produce more CO2, which not only causes more defects on the coke surface to adsorb NO, but also reduces the oxygen partial pressure to promote the reaction between CO and NO.
CONCLUSIONS
In the process of coke combustion and sintering, the effect of CeO2 added to coke on NO emission reduction was studied. It is found that 1–2% CeO2 addition during coke combustion can reduce the total amount of NOx emissions during coke combustion. More than 3% of CeO2 addition promotes NOx emissions during the combustion of coke. At the same time, the addition of 2% CeO2 does not reduce the yield of sintered ore, but increases the drum strength of the sintered ore. The reason about CeO2 modified coke reduces NO release during coke combustion and sintering is that CeO2 promotes the reaction between CO and NO and between C and NO.
REFERENCES
Kondragunta, S., Dickerson, R.R., Stenchikov, G., et al., The radiative effects of aerosols on photochemical smog: measurements and modeling, 2000. http://citeseerx.ist.psu.edu/viewdoc/download?doi= 10.1.1.39.4485&rep=rep1&type=pdf.
Crutzen, P.J., The influence of nitrogen oxides on the atmospheric ozone content, Q. J. R. Meteorol. Soc., 2010, vol. 96, no. 408, pp. 320–325.
Oman, J., Kuštrin, I., Bole, I., et al., The reduction of nitrogen oxide emissions by the boiler firing system reconstruction at the Power Plant Ljubljana, Strojniski Vestn., 2005, vol. 51, no. 5, pp. 240–251.
Yokoyama, T., Nishinomiya, S., and Matsuda, H., Nitrous oxide emissions from fossil fuel fired power plants, Environ. Sci. Technol., 2002, vol. 25, no. 2, pp. 347–348.
Wendt, J.O.L., Linak, W.P., Groff P.W., et al., Hybrid SNCR-SCR technologies for NOx control: modeling and experiment, AIChE J., 2001, vol. 47, no. 11, pp. 2603–2617.
Fiskum, A., Calculation of NOx formation in a swirl burner, MSc Thesis, Trondheim: Norw. Univ. Sci. Technol., 2008.
Gómez-García, M.A., Pitchon, V., and Kiennemann, A., Pollution by nitrogen oxides: an approach to NOx abatement by using sorbing catalytic materials, Environ. Int., 2005, vol. 31, no. 3, pp. 445–467.
Lee, M.S. and Shim, S.C., Influence of lime/limestone addition on the SO2 and NO formation during the combustion of coke pellet, ISIJ Int., 2004, vol. 44, no. 3, pp. 470–475.
Chen, Y., Guo, Z., and Wang, Z., Influence of CeO2 on NOx emission during iron ore sintering, Fuel Process. Technol., 2009, vol. 90, nos. 7–8, pp. 933–938.
Gong, X., Guo, Z., and Wang, Z., Reactivity of pulverized coals during combustion catalyzed by CeO2 and Fe2O3, Combust. Flame, 2010, vol. 157, no. 2, pp. 351–356.
Gong, X., Guo, Z., and Zhi, W., Variation on anthracite combustion efficiency with CeO2 and Fe2O3 addition by Differential Thermal Analysis (DTA), Energy, 2010, vol. 35, no. 2, pp. 506–511.
Funding
This work was supported by the Natural Scientific Foundation of China (Grants U1710114, 21878001, 21776001, and 21808002), the National Key Research and Development Program of China (Grant 2018YFB0604600), and the Natural Science Foundation of Anhui Provincial Education Department (Grants KJ2018A0058 and KJ2018A0064). Authors are also appreciative for the financial support from the Provincial Innovative Group for Processing and Clean Utilization of Coal Resource.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Song Han, Lin Dong, Zhiping Lei et al. Study on NOx Emission Reduction in Coke Combustion and Sintering Process. Coke Chem. 62, 585–592 (2019). https://doi.org/10.3103/S1068364X19120093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X19120093