Abstract—
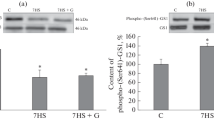
Molecular mechanisms initiating the development of atrophic changes in a mammalian postural muscle remain unclear. We have suggested that AMP-activated protein kinase (AMPK) may play an important role in the regulation of anabolic and catabolic signaling pathways in postural muscle at the initial stage of mechanical unloading, since skeletal muscle inactivation can lead to changes in the balance of intramuscular high-energy phosphates. Beta-guanidinopropionic acid (β-GPA) can act as a modulator of the balance of high-energy phosphates, application of which can reduce the content of intracellular high-energy phosphates. The purpose of this study was to assess signaling pathways of protein synthesis and degradation in rat soleus muscle after preliminary treatment with β-GPA followed by 1-day hindlimb unloading. The experiments were performed on male Wistar rats, which were divided into the following groups: (1) vivarium control (C); (2) vivarium control + β-GPA (C + GPA); (3) 1-day hindlimb unloading (HU); and (4) 1-day hindlimb unloading + β-GPA (HU + GPA). β-GPA was administered daily by intraperitoneal injections (400 mg/kg). The content of the key signaling proteins (AMPK, ACC, p70S6K, 4E-BP1, and FOXO3) was determined by gel electrophoresis followed by immunoblotting. The expression of mRNA of ubiquitin ligases (MuRF1 and MAFbx) was evaluated by real-time PCR. A prevention of reduction in phosphorylation of AMPK, ACC and 4E-BP1 and the return to control values of the increased level of p70S6K phosphorylation were observed in the group with 1-day unloading and β-GPA pretreatment. The phosphorylation of AKT and FOXO3, as well as the expression levels of MuRF1 and MAFbx in the HU + GPA group did not differ from the HU group. The obtained data indicate that the AMPK activity may play an important role in the modulation of anabolic mTORC1-signaling in rat soleus muscle at the early stage of simulated microgravity.





Similar content being viewed by others
REFERENCES
Fitts R. H., Riley D. R., Widrick J. 2000. Physiology of a microgravity environment invited review: Microgravity and skeletal muscle. J. Appl. Physiol.89, 823–839.
Ohira Y., Saito K., Wakatsuki T., Yasui W., Suetsugu T., Nakamura K., Tanaka H., Asakura T. 1994. Responses of beta-adrenoceptor in rat soleus to phosphorus compound levels and/or unloading. Am. J. Physiol. 266, 1257–1262.
Wakatsuki T., Ohira Y., Yasui W., Nakamura K., Asakura T., Ohno H., Yamamoto M. 1994. Responses of contractile properties in rat soleus to high-energy phosphates and/or unloading. Jpn. J. Physiol.44, 193–204.
Hardie D.G. 2011. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908.
Carling D. 2017. AMPK signalling in health and disease. Curr. Opin. Cell. Biol. 45, 31–37.
Thomson D.M. 2018. The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int. J. Mol. Sci.19 (10), pii E3125. https://doi.org/10.3390/ijms19103125
Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., Yancopoulos G.D. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo.Nat. Cell Biol. 3, 1014–1019.
Glass D.J. 2003. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol.5, 87–90.
Krawiec B.J., Nystrom G.J., Frost R.A., Jefferson L.S., Lang C.H. 2007. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am. J. Physiol. Enocrinol. Metab.292, 1555–1567,
Nakashima K., Yakabe Y. 2007. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem.71, 1650–1656.
Mahanna D.A., Fitch C.D., Fischer V.W. 1980. Effects of beta-guanidinopropionic acid on murine skeletal muscle. Exp. Neurol. 68, 114–121.
Oudman I., Clark J.F., Brewster L.M. 2013. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: A systematic review. PLoS One. 8, e52879.
Williams D.B., Sutherland L.N., Bomhof M.R., Basaraba S.A.U., Thrush A.B., Dyck D.J., Field C.J., Wright D.C. 2009. Muscle-specific differences in the response of mitochondrial proteins to β-GPA feeding: An evaluation of potential mechanisms. Am. J. Physiol. Endocrinol. Metab.296, 1400–1408.
Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J., Shulman G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. 2002. Proc. Natl. Acad. Sci. USA. 99, 15983–15987.
Morey-Holton E., Globus R. 2002. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol.92, 1367–1377.
Mirzoev T., Tyganov S., Vilchinskaya N., Lomonosova Y., Shenkman B. 2016. Key markers of mTORC1-dependent and mTORC1-independent signaling pathways regulating protein synthesis in rat soleus muscle during early stages of hindlimb unloading. Cell. Physiol. Biochem. 39, 1011–1020.
Vilchinskaya N.A., Mochalova E.P., Nemirovskaya T.L., Mirzoev T.M., Turtikova O.V., Shenkman B.S. 2017. Rapid decline in MyHC I (beta) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J. Physiol. 595, 7123–7134.
Chibalin A.V., Benziane B., Zakyrjanova G.F., Kravtsova V.V., Krivoi I.I. 2018. Early endplate remodeling and skeletal muscle signaling events following rat hindlimb suspension. J. Cell. Physiol. 233, 6329–6336.
Mochalova E.P., Belova S.P., Shenkman B.S., Nemirovskaya T.L. 2018. The role of transcription factors FoxO3 and myogenin in the regulation of E3-ligases MuRF-1 and MAFbx expression in rat soleus at the early stage of disuse atrophy. Biol. Membrany (Rus.).35, 443–447.
Vilchinskaya N.A., Mochalova E.P., Belova S.P., Shenkman B.S. 2016. Dephosphorylation of AMP-activated protein kinase in a postural muscle: A key signaling event on the first day of functional unloading. Biophysics. 61, 1019–1061.
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation (project no. 17-75-20 152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that there is no conflict of interest.
All procedures with animals were approved by the Biomedicine Ethics Committee of the Institute of Biomedical Problems of the Russian Academy of Sciences/Physiology section of the Russian Bioethics Committee (protocol no. 447 of March 28, 2017). All experiments were performed in accordance with the guidelines and recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Vilchinskaya, N.A., Mochalova, E.P., Paramonova, I.I. et al. The Effect of β-GPA on the Markers of Anabolic and Catabolic Signaling Pathways in Rat Soleus Muscle at the Initial Stage of Hindlimb Unloading. Biochem. Moscow Suppl. Ser. A 14, 1–6 (2020). https://doi.org/10.1134/S1990747819060102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819060102