Abstract
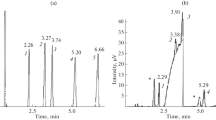
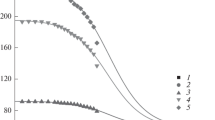
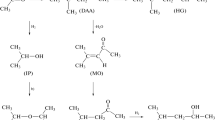
Characteristic features of the gas chromatographic separation of the keto and enol tautomeric forms of ethyl acetoacetate on a capillary column with a BPX-1 standard nonpolar polydimethylsiloxane stationary phase are considered. It is confirmed that the chromatograms of a mixture of tautomers have a specific profile, i.e., a plateau between the peaks of the tautomers, which corresponds to the reversible keto \( \rightleftarrows \) enol transformation during separation. It is shown that the enol and keto forms of ethyl acetoacetate have different coefficients of the temperature dependence of the gas chromatographic retention indices (0.19 ± 0.03 and 0.02 ± 0.02, respectively). It is found there is no dependence of the relative peak areas of the tautomers on the nature of the solvent (polar ethyl alcohol and nonpolar hexane) at different temperatures; i.e., such ratios predominantly reflect the position of the keto \( \rightleftarrows \) enol equilibrium in the vapor phase of the injector of the chromatograph. It is concluded that this results in similar values of the thermodynamic parameters (standard enthalpy and entropy) of the tautomeric equilibrium determined upon the dosing of samples in different solvents. Possible distortions of the results due to the effects of discriminating between the compositions of the samples injected into capillary columns with gas flow splitting are discussed. An impurity in a sample of ethyl acetoacetate after long-term storage is identified as ethyl 2-hydroxy-3-oxobutanoate, the product of oxidation by dissolved atmospheric oxygen.





Similar content being viewed by others
REFERENCES
V. I. Minkin, L. P. Olekhnovich, and Yu. A. Zhdanov, Molecular Design of Tautomeric Compounds (Kluwer, Dordrecht, Boston, Tokio, 1958). https://doi.org/10.1007/978-94-009-1429-2
J. N. Spencer, E. S. Holmboe, M. R. Kirshembaum, D. W. Firth, and P. B. Pinto, Can. J. Chem. 68, 1178 (1982).
P. Umnahanant and J. S. Chickos, J. Chem. Eng. Data 50, 1720 (2005). https://doi.org/10.1021/je050179z
D. Antic, Thermo Scientific Application Note No. AN52327 (2017).
M. T. Rogers and J. L. Burdett, Can. J. Chem. 43, 1516 (1965).
D. Tiess and Z. Wiess, Willhelm-Pieck-Univ. Rostock Math. Naturwiss. Reiche 33, 6 (1984).
T. L. Peppard, J. Agric. Food Chem. 40, 257 (1992). https://doi.org/10.1021/jf00014a018
E. Tudor, J. Chromatogr., A 779, 287 (1997). https://doi.org/10.1016/S0021-9673(97)00453-6
E. Tudor, D. Moldovan, and N. Zarna, Rev. Roum. Chim. 44, 665 (1999).
M. J. Jordan, K. L. Goodner, and P. E. Shau, J. Agric. Food Chem. 50, 1523 (2002). https://doi.org/10.1021/jf011077p
M. Adamova, A. Orinak, and L. Halas, J. Chromatogr., A 1087, 131 (2005). https://doi.org/10.1016/j.chroma.2005.01.003
J. A. Pino, J. Mesa, Y. Munos, M. P. Marti, and R. Marbot, J. Agric. Food Chem. 53, 2213 (2005).https://doi.org/10.1021/jf0402633
F. Bianchi, M. Careri, A. Mangia, and M. Musci, J. Sep. Sci. 39, 563 (2007).
The NIST 17 Mass Spectral Library (NIST17/2017/EPA/NIH), Software/Data Version (NIST17), NIST Standard Reference Database, Number 69 (Natl. Inst. Standards Technol., Gaithersburg, MD, 2017). http://webbook.nist.gov. Accessed October 2019.
M. Masur, H. F. Grutzmascher, H. Munster, and H. Budzikieicz, Org. Mass Spectrom. 22, 493 (1987).
P. E. Allegretti, M. M. Schiavoni, H. E. di Loreto, and J. J. P. Furlong, and C. O. Della Vedova, J. Mol. Struct. 560, 327 (2001).
S. J. Ruggiero and V. M. Luaces, J. Chem. Educ. 65, 629 (1988). https://doi.org/10.1021/ed065p629
V. Krishman, Inventions 4, 15 (2019). https://doi.org/10.3390/inventions4010013
T. A. Kornilova, A. I. Ukolov, R. R. Kostikov, and I. G. Zenkevich, Rapid Commun. Mass Spectrom. 27, 461 (2013). https://doi.org/10.1002/rcm.6457
I. G. Zenkevich and N. E. Podol’skii, Anal. Kontrol’ 21 (2), 125 (2017). https://doi.org/10.15825/analitika.2017.21.2.002
P. J. Skrdla, V. Antomucci, and C. Lindemann, J. Chromatogr. Sci. 39, 431 (2001).
Guide on Gas Chromatography, Ed. by E. Leibnitz and H. G. Struppe (Akad. Verlag, Leipzig, 1966; Mir, Moscow, 1988).
K. Grob and H. P. Neukom, J. Chromatogr., A 236, 297 (1982). https://doi.org/10.1016/S0021-9673(00)84878-5
I. G. Zenkevich and D. A. Olisov, Labor. Pr-vo, No. 2, 92 (2018).
I. G. Zenkevich and E. Leleev, Anal. Kontrol’ 23, 110 (2019). https://doi.org/10.15826/analitika.2019.23.1.012
I. G. Zenkevich and D. A. Olisov, J. Anal. Chem. 74 (Suppl. 1), S32 (2019). https://doi.org/10.1134/S1061934819070190
I. G. Zenkevich and V. V. Ioffe, The Interpretation of Mass-Spectra of Organic Compounds (Khimiya, Leningrad, 1986) [in Russian].
I. G. Zenkevich and V. M. Lukina, Anal. Kontrol’ 23, 410 (2019). https://doi.org/10.15826/analitika.2019.23.3.009
M. C. Hamming and N. G. Foster, Interpretation of Mass Spectra of Organic Compounds (Academic, New York, 1979).
I. G. Zenkevich, M. Moeder, G. Koeller, and S. Schrader, J. Chromatogr., A 1025, 227 (2004). https://doi.org/10.1016/j.chroma.2003.10.106
I. G. Zenkevich and A. I. Ukolov, Russ. J. Gen. Chem. 81, 1818 (2011). https://doi.org/10.1134/1070363211090143
I. G. Zenkevich and A. I. Ukolov, Mass-Spektrom. 8, 264 (2011). https://doi.org/10.1134/S1061934812130114
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Lukina, V.M. Characteristic Features of the Gas Chromatographic Separation of Tautomers of Ethyl Acetoacetate. Russ. J. Phys. Chem. 94, 1214–1223 (2020). https://doi.org/10.1134/S0036024420060357
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420060357