Abstract
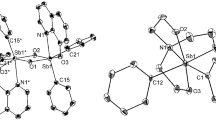
Tetraarylantimony carboxylates Ph4SbOC(O)R (R = CF2Br (I), CF2CF2CF3 (II)), (4‑MeC6Н4)4SbOC(O)CF2CF3 (III), and (4-MeC6Н4)4SbOC(O)CF2CF2CF3 (IV) have been synthesized by the reaction between pentaarylantimony Ar5Sb (Ar = Ph, 4-MeC6H4) and fluorine-containing carboxylic acids. Despite an excess amount of carboxylic acid in the reaction mixture, it does not form any solvates Ar4SbOC(O)R ⋅ HOC(O)R with tetraarylantimony carboxylates. X-ray diffraction shows that the Sb atoms in complexes I–IV have differently distorted trigonal bipyramidal coordination. Crystals of complexes III and IV contain two types of crystallographically independent molecules. The axial СSbO angles in complexes I, II, III, and IV are 175.32(14)°, 178.87(8)°, 178.56(13)° and 178.33(10)°, 178.16(13)°, and 179.58(15)°, respectively. The Sb–O and Sb–С bond lengths are 2.333(3) and 2.106(4)–2.151(5) Å in I, 2.340(2) and 2.101(2)–2.154(3) Å in II, 2.364(3), 2.411(3), and 2.096(4)–2.159(4) Å in III, and 2.376(3), 2.367(3), and 2.105(4)–2.161(4) Å in IV. The intramolecular Sb⋅⋅⋅O distances with the carbonyl oxygen atom are 3.506(4) Å (I), 3.517(6) Å (II), 3.565(6) Å (III), and 3.527(6) Å (IV), being ~0.2 Å smaller than the sum of Sb and O van der Waals radii. The second carbonyl oxygen atoms in crystals of complexes III and IV do not participate in the coordination with the central metal atom.




Similar content being viewed by others
REFERENCES
S. K. Hadjikakou, I. I. Ozturk, C. N. Banti, et al., J. Inorg. Biochem. 153, 293 (2015). https://doi.org/10.1016/j.jinorgbio.2015.06.006
M. I. Ali, M. K. Rauf, A. Badshah, et al., Dalton Trans. 42, 16733 (2013). https://doi.org/10.1039/C3DT51382C
X. Y. Zhang, L. S. Cui, X. Zhang, et al., J. Mol. Struct. 1134, 742 (2017). https://doi.org/10.1016/j.molstruc.2017.01.039
A. V. Gushchin, E. V. Grunova, D. V. Moiseev, et al., Izv. Russ. Akad. Nauk, Ser. Khim., No. 6, 1302 (2003).
L. Quan, H.-D. Yin, J.-C. Cui, et al., J. Organomet. Chem. 694, 3708 (2009). https://doi.org/10.1016/j.jorganchem.2009.07.040
L. Wen, H. Yin, W. Li, et al., Inorg. Chim. Acta 363, 676 (2010). https://doi.org/10.1016/j.ica.2009.11.022
J.-S. Li, Y.-Q. Ma, J.-R. Cui, et al., Appl. Organomet. Chem. 15, 639 (2001). https://doi.org/10.1002/aoc.200
Y.-Q. Ma, J.-S. Li, Z.-A. Xuan, et al., J. Organomet. Chem. 620, 235 (2001). https://doi.org/10.1016/S0022-328X(00)00799-3
H.-D. Yin, L.-Y. Wen, J.-C. Cui, et al., Polyhedron 28, 2919 (2009). https://doi.org/10.1016/j.poly.2009.06.065
J.-S. Li, R.-C. Liu, X.-B. Chi, et al., Inorg. Chim. Acta 357, 2176 (2004). https://doi.org/10.1016/j.ica.2003.12.012
V. V. Sharutin and O. K. Sharutina, Russ. J. Inorg. Chem. 62, 905 (2017). https://doi.org/10.1134/S003602361707021X
V. V. Sharutin, O. K. Sharutina, and V. S. Senchurin, Russ. J. Coord. Chem. 40, 109 (2014). https://doi.org/10.1134/S1070328414020109
V. V. Sharutin, O. K. Sharutina, and A. R. Kotlyarov, Russ. J. Inorg. Chem. 60, 465 (2015). https://doi.org/10.1134/S0036023615040221
V. V. Sharutin, O. K. Sharutina, Yu. O. Gubanova, et al., Russ. J. Inorg. Chem. 64, 1138 (2019). https://doi.org/10.1134/S0036023619090195
SMART and SAINT-Plus: Data Collection and Processing Software for the SMART System, Versions 5.0 (Bruker, Madison, Wisconsin, USA, 1998).
SHELXTL/PC: An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Versions 5.10 (Bruker, Madison, Wisconsin, USA, 1998).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Zh. Obshch. Khim. 66, 1755 (1996).
H. Schmidbaur and K. H. Mitschke, Angew. Chem., No. 83, 149 (1971). https://doi.org/10.1002/zaac.19713860204
S. S. Batsanov, Russ. J. Inorg. Chem. 36, 1694 (1991).
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Inorg. Chem. 53, 1110 (2008). https://doi.org/10.1134/S0036023608070206
S. P. Bone and D. B. Sowerby, Phosphorus, Sulfur Silicon Relat. Elem. 45, 23 (1989). https://doi.org/10.1080/10426508908046072
Funding
This work was financially supported within state task no. 4.6151.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K., Efremov, A.N. et al. Fluorine-Containing Tetraarylantimony Carboxylates: Synthesis and Structure. Russ. J. Inorg. Chem. 65, 502–506 (2020). https://doi.org/10.1134/S0036023620040178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620040178