Abstract
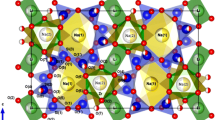
Thermal behavior of catapleiite, ideally Na2ZrSi3O9 ⋅ 2H2O, has been investigated with the X-ray diffraction, electroconductivity measurements using impedance spectroscopy, dynamic scanning calorimetry, thermal gravimetry, and infrared spectroscopy. The high-temperature transformation product of catapleiite obtained after annealing of catapleiite at 1000°C has been studied. This is a new microporous zirconosilicate, ideally Na6Zr3[Si9O27], representing a new structural type. It is hexagonal, P63/mcm, a = 11.5901(9), c = 9.9546(9) Å, V = 1158.05(16) Å3. Its crystal structure model has been obtained using single-crystal X-ray diffraction data and refined by the Rietveld method on the basis of the powder X-ray diffraction data (R = 3.87%). The structure is based on the heteropolyhedral framework, which substantially differs from that of catapleiite and is built by nine-member rings Si9O27 formed by SiO4 tetrahedra linked with isolated ZrO6 octahedra. Extraframework Na cations are located in broad channels of the framework and between the tetrahedral Si9O27 rings.





Similar content being viewed by others
REFERENCES
Baranov, A.I., Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Review, Crystal. Rept., 2003, vol. 48, no. 6, pp. 1012–1037.
Brunowsky, B., Die Struktur des Katapleits (Na2ZrSi3O9 ⋅ 2H2O), Acta Phys. URSS, 1936, vol. 5, no. 6, pp. 863–892.
Chao, G.Y., Rowland, J.R., and Chen, T.T., The crystal structure of catapleiite, Abstr. Programs -Geol. Soc. Amer, 1973, p. 572.
Chukanov, N.V. and Pekov, I.V., Infrared spectroscopy of acid salts. I. The silicate minerals, Zap. Ross. Mineral.O-va, 2012, vol. 141, no. 3, pp. 129–143.
Fleet, M.E., Sodium tetrasilicate: a complex high-pressure framework silicate (Na6Si3[Si9O27]), Am. Mineral., 1996, vol. 81, pp. 1105–1110.
Ilyushin, G.D., and Dem’yanets, L.N., Crystal-structural features of ion transport in new OD structures: catapleiite Na2ZrSi3O9 ⋅ 2H2O and hilairite Na2ZrSi3O9 ⋅ 3H2O, Kristallografiya, 1988, vol. 33, pp. 383–387.
Ilyushin, G.D., Voronkov, A.A., Ilyukhin, V.V., Nevsky, N.N., and Belov, N.V., The crystal structure of the native monoclinic catapleiite Na2ZrSi3O9 ⋅ 2H2O, Dokl. Akad. Nauk SSSR, 1981, vol. 260, pp. 623–627.
Johnsen, O. and Grice, J.D., The crystal chemistry of the eudialyte group, Can. Mineral., 1999, vol. 37, pp. 865–891.
Merlino, S., Pasero, M., Bellezza, M., Pushcharovsky, D.Yu., Gobechia, E.R., Zubkova, N.V., and Pekov, I.V., Crystal structure of calcium catapleiite, Can. Mineral., 2004, vol. 42, pp. 1037–1045.
Petříček V., Dušek M., and Palatinus, L., Jana2006. Structure Determination Software Programs, Praha: Instit. Phys., 2006.
Sheldrick, G.M., A short history of SHELX, Acta Crystallogr, 2008, vol. A64, pp. 112–122.
Sitarza, M., Handke, M., Mozgawa, W., Galuskin, E., and Galuskina, I., The non-ring cations influence on silicooxygen ring vibrations, J. Molecular Structure, 2000, vol. 555, pp. 357–362.
Yakubovich, O.V., Karimova, O.V., Ivanova, A.G., Yapaskurt, V.O., Chukanov, N.V., and Kartashov, P.M., Ordering of cations in the voids of the anionic framework of the crystal structure of catapleiite. Crystal.Rept., 2013, vol. 58, pp. 401–411.
Zubkova, N.V., Comparative Crystal Chemistry of New and Rare Zirconosilicates, Silicates of Alkaline and Alkaline Earth Elements and Minerals with Isolated Tetrahedral and Triangular Oxocomplexes, Doctoral Dissertation in Geology and Mineralogy, Moscow: MSU, 2012.
Zubkova, N.V. and Pushcharovsky, D.Yu., New Data on the crystal structures of natural zirconosilicates: structure refinements and ion-exchange behavior, Zeit. Kristallogr, 2008, vol. 223, pp. 98–108.
Zubkova, N.V., Pekov, I.V., Turchkova, A.G., Pushcharovskii, D.Yu., Merlino, S., Peasero, M., and Chukanov, N.V., Crystal structures of potassium-exchanged forms of catapleiite and hilairite. Crystal.Rept., 2007, vol. 52, pp. 65–70.
Funding
This study has been supported by the Russian Foundation for Basic Research (project no 18-05-00332) in regard to the powder X-ray diffraction study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by I. Baksheev
Rights and permissions
About this article
Cite this article
Ksenofontov, D.A., Grebenev, V.V., Zubkova, N.V. et al. Behavior of Catapleiite under Heating and Crystal Structure of its High-Temperature Transformation Product, a New Phase Na6Zr3[Si9O27] with Nine-membered Rings of SiO4 Tetrahedra. Geol. Ore Deposits 61, 696–705 (2019). https://doi.org/10.1134/S1075701519070080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1075701519070080