Abstract—
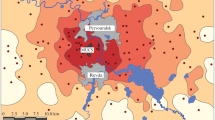
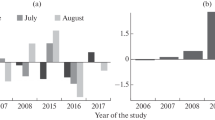
The type of diaspore dispersal may have a significant effect on the vulnerability of herbaceous plants to anthropogenic transformation of ecosystems. However, there is still no unified opinion on the relationship between the type of diaspore dispersal and the processes of extinction and colonization. Here, descriptions of vegetation performed in the zone of impact from the Middle Ural Copper Smelter in the periods of high and low emission levels (1995–1998 and 2014–2016, respectively) have been analyzed to test two hypotheses: (1) the type of diaspore dispersal has an effect on plant vulnerability, with the species whose diaspores are dispersed farther being less vulnerable to habitat transformation; and (2) irrespective of the type of dispersal, recolonization is less intense under high than under low pollution levels. It has been found that the species richness of all plant groups highly varies in space: it decreases sharply in the zone of heavy toxic loads, but localities with high species richness occur even under conditions of heavy pollution. With the increasing pollution level, the species diversity within plant groups on a mesoscale (hundreds of meters) decreases more strongly than that on a macroscale (kilometers). Species with different types of diaspore dispersal differ from each other both in sensitivity to pollution and in the capacity for recolonization after reduction of emissions, but the relationship between vulnerability and the distance of diaspore dispersal is ambiguous. High sensitivity to pollution and low recolonization capacity have been revealed in species characterized by either the lowest (autochores) or the highest dispersal distance (epi- and endozoochores), with anemochores being the least vulnerable. The diversity of most groups in the zone of heavy pollution has remained low over the past 20 years; some positive shifts have occurred in myrmecochores and typical anemochores, with the dynamics of the latter being independent of pollution level.


Similar content being viewed by others
REFERENCES
Haddad, N.M., Brudvig, L.A., Clobert, J., et al., Habitat fragmentation and its lasting impact on Earth’s ecosystems, Sci. Adv., 2015, vol. 1, no. 2.
Kolk, J. and Naaf, T., Herb layer extinction debt in highly fragmented temperate forests: Completely paid after 160 years?, Biol. Conserv., 2015, vol. 182, pp. 164–172.
Cadotte, M.W., Dinnage, R., and Tilman, D., Phylogenetic diversity promotes ecosystem stability, Ecology, 2012, vol. 93, no. 8, pp. 223–233.
Cardinale, B.J., Duffy, J.E., Gonzalez, A., et al., Biodiversity loss and its impact on humanity, Nature, 2012, vol. 486, no. 7401, pp. 59–67.
Bukvareva, E.N., Rol’ nazemnykh ekosistem v regulyatsii klimata i mesto Rossii v postkiotskom protsesse (Role of Terrestrial Ecosystems in Climate Regulation, and the Place for Russia in the Post-Kyoto Process), Moscow: Litres, 2017.
Gilliam, F.S., The ecological significance of the herbaceous layer in temperate forest ecosystems, AIBS Bull., 2007, vol. 57, no. 10, pp. 845–858.
Moore, P.T., Van Miegroet, H., and Nicholas, N.S., Relative role of understory and overstory in carbon and nitrogen cycling in a southern Appalachian spruce–fir forest, Can. J. For. Res., 2007, vol. 37, no. 12, pp. 2689–2700.
Milcu, A., Thebault, E., Scheu, S., and Eisenhauer, N., Plant diversity enhances the reliability of belowground processes, Soil Biol. Biochem., 2010, vol. 42, no. 12, pp. 2102–2110.
Mikhailova, N.V., Bogdanova, N.E., and Mikhailov, A.V., The rate of area colonization by nemoral herbaceous species: A model approach, Byull. Mosk. O-va. Ispyt. Prir.,Otd. Biol., 2006, vol. 111, no. 1, pp. 37–44.
Baeten, L., Jacquemyn, H., Van Calster, H., et al., Low recruitment across life stages partly accounts for the slow colonization of forest herbs, J. Ecol., 2009, vol. 97, no. 1, pp. 109–117.
Brunet, J., De Frenne, P., Holmström, E., and Mayr, M.L., Life-history traits explain rapid colonization of young post-agricultural forests by understory herbs, Forest Ecol. Manag., 2012, vol. 278, pp. 55–62.
Nordén, B., Dahlberg, A., Brandrud, T.E., et al., Effects of ecological continuity on species richness and composition in forests and woodlands: A review, Ecoscience, 2014, vol. 21, no. 1, pp. 34–45.
Myers, J.A. and Harms, K.E., Seed arrival, ecological filters, and plant species richness: A meta-analysis, Ecol. Lett., 2009, vol. 12, no. 11, pp. 1250–1260.
Vittoz, P. and Engler, R., Seed dispersal distances: A typology based on dispersal modes and plant traits, Bot. Helv., 2007, vol. 117, no. 2, pp. 109–124.
Bullock, J.M., Mallada González, L., Tamme, R., et al., A synthesis of empirical plant dispersal kernels, J. Ecol., 2017, vol. 105, no. 1, pp. 6–19.
Hemrova, L., Bullock, J.M., Hooftman, D.A.P., et al., Drivers of plant species’ potential to spread: The importance of demography versus seed dispersal, Oikos, 2017, vol. 126, no. 10, pp. 1493–1500.
Levina, R.E., Morfologiya i ekologiya plodov (The Morphology and Ecology of Fruits), Leningrad: Nauka, 1987.
Nathan, R., Schurr, F.M., Spiegel, O., et al., Mechanisms of long-distance seed dispersal, Trends Ecol. Evol., 2008, vol. 23, no. 11, pp. 638–647.
Higgins, S.I., Nathan, R., and Cain, M.L., Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal?, Ecology, 2003, vol. 84, no. 8, pp. 1945–1956.
Ash, J.D., Givnish, T.J., and Waller, D.M., Tracking lags in historical plant species' shifts in relation to regional climate change, Glob. Change Biol., 2017, vol. 23, no. 3, pp. 1305–1315.
Fröborg, H. and Eriksson, O., Local colonization and extinction of field layer plants in a deciduous forest and their dependence upon life history features, J. Veget. Sci., 1997, vol. 8, no. 3, pp. 395–400.
Ozinga, W.A., Römermann, C., Bekker, R.M., et al., Dispersal failure contributes to plant losses in NW Europe, Ecol. Lett., 2009, vol. 12, no. 1, pp. 66–74.
Marini, L., Bruun, H.H., Heikkinen, R.K., et al., Traits related to species persistence and dispersal explain changes in plant communities subjected to habitat loss, Divers. Distrib., 2012, vol. 18, no. 9, pp. 898–908.
García, C., Klein, E.K., and Jordano, P., Dispersal processes driving plant movement: Challenges for understanding and predicting range shifts in a changing world, J. Ecol., 2017, vol. 105, no. 1, pp. 1–5.
Flinn, K.M., Microsite-limited recruitment controls fern colonization of post-agricultural forests, Ecology, 2007, vol. 88, no. 12, pp. 3103–3114.
Paal, T., Kütt, L., Lõhmus, K., and Liira, J., Both spatiotemporal connectivity and habitat quality limit the immigration of forest plants into wooded corridors, Plant Ecol., 2017, vol. 218, no. 4, pp. 417–431.
Thomson, F.J., Moles, A.T., Auld, T.D., and Kingsford, R.T., Seed dispersal distance is more strongly correlated with plant height than with seed mass, J. Ecol., 2011, vol. 99, no. 6, pp. 1299–1307.
Albert, A., Auffret, A.G., Cosyns, E., et al., Seed dispersal by ungulates as an ecological filter: A trait-based meta-analysis, Oikos, 2015, vol. 124, no. 9, pp. 1109–1120.
Sonkoly, J., Deak, B., Valko, O., et al., Do large-seeded herbs have a small range size? The seed mass-distribution range trade-off hypothesis, Ecol. Evol., 2017, vol. 7, no. 24, pp. 11204–11212.
Beckman, N.G., Bullock, J.M., and Salguero-Gomez, R., High dispersal ability is related to fast life-history strategies, J. Ecol., 2018, vol. 106, no. 4, pp. 1349–1362.
Evstigneev, O.I., Korotkov, V.N., Murashev, I.A., and Voevodin, P.V., Zoochory and peculiarities of forest community formation: A review, Russ. J. Ecosyst. Ecol., 2017, no. 1, pp. 1–16.
Forey, E., Barot, S., Decaens, T., et al., Importance of earthworm–seed interactions for the composition and structure of plant communities: A review, Acta Oecol., 2011, vol. 37, no. 6, pp. 594–603.
Lepková, B., Horčičková, E., and Vojta, J., Endozoochorous seed dispersal by free-ranging herbivores in an abandoned landscape, Plant Ecol., 2018, vol. 219, no. 9, pp. 1127–1138.
Penn, H.J. and Crist, T.O., From dispersal to predation: A global synthesis of ant–seed interactions, Ecol. Evol., 2018, vol. 8, no. 18, pp. 9122–9138.
Lustenhouwer, N., Moran, E.V., and Levine, J.M., Trait correlations equalize spread velocity across plant life histories, Glob. Ecol. Biogeogr., 2017, vol. 26, no. 12, pp. 1398–1407.
Ehrlén, J. and Eriksson, O., Dispersal limitation and patch occupancy in forest herbs, Ecology, 2000, vol. 81, no. 6, pp. 1667–1674.
Verheyen, K., Hermy, M., and Thompson, K., Recruitment and growth of herb-layer species with different colonizing capacities in ancient and recent forests, J. Veget. Sci., 2004, vol. 15, no. 1, pp. 125–134.
Meadows, M. and Watmough, S.A., An assessment of long-term risks of metals in Sudbury: A critical loads approach, Water Air Soil Pollut., 2012, vol. 223, no. 7, pp. 4343–4354.
Trubina, M.R., Vorobeichik, E.L., Khantemirova, E.V., et al., Dynamics of forest vegetation after the reduction of industrial emissions: Fast recovery or continued degradation?, Dokl. Biol. Sci., 2014, vol. 458, pp. 302–305.
Vorobeichik, E.L. and Kaigorodova, S.Yu., Long-term dynamics of heavy metals in the upper horizons of soils in the region of a copper smelter impacts during the period of reduced emission, Euras. Soil Sci., 2017, vol. 50, no. 8, pp. 977–990.
Trubina, M.R. and Vorobeichik, E.L., Severe industrial pollution increases the β-diversity of plant communities, Dokl. Biol. Sci., 2012, vol. 442, pp. 17–19.
Prokaev, V.I., Fiziko-geograficheskoe raionirovanie Sverdlovskoi oblasti (Physiographic Zoning of Sverdlovsk Oblast), Sverdlovsk: Sverdlovsk. Ped. Inst., 1976.
Metzger, J.P., Martensen, A.C., Dixo, M., et al., Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region, Biol. Conserv., 2009, vol. 142, no. 6, pp. 1166–1177.
Hylander, K. and Ehrlén, J., The mechanisms causing extinction debts, Trends Ecol. Evol., 2013, vol. 28, no. 6, pp. 341–346.
Ernst, W.H.O., Evolution of metal tolerance in higher plants, For. Snow Landsc. Res., 2006, vol. 80, no. 3, pp. 251–274.
Dulya, O.V., Mikryukov, V.S., and Vorobeichik, E.L., Strategies of adaptation to heavy metal pollution in Deschampsia caespitosa and Lychnis flos-cuculi: Analysis based on dose–response relationship, Russ. J. Ecol., 2013, vol. 44, no. 4, pp. 271–281.
Ginocchio, R., Carvallo, G., Toro, I., et al., Micro-spatial variation of soil metal pollution and plant recruitment near a copper smelter in central Chile, Environ. Pollut., 2004, vol. 127, no. 3, pp. 343–352.
Kataev, G.D., Suornela, J., and Palokangas, P., Densities of microtine rodents along a pollution gradient from a copper–nickel smelter, Oecologia, 1994, vol. 97, no. 4, pp. 491–498.
Bel’skii, E. and Lyakhov, A., Response of the avifauna to technogenic environmental pollution in the southern taiga zone of the Middle Urals, Russ. J. Ecol., 2003, vol. 34, no. 3, pp. 181–187.
Mukhacheva, S.V., Spatiotemporal population structure of the bank vole in a gradient of technogenic environmental pollution, Russ. J. Ecol., 2007, vol. 38, no. 3, pp. 161–167.
Mukhacheva, S.V., Davydova, Yu.A., and Kshnyasev, I.A., Responses of small mammal community to environmental pollution by emissions from a copper smelter, Russ. J. Ecol., 2010, vol. 41, no. 6, pp. 513–518.
Eeva, T., Belskii, E., Gilyazov, A.S., and Kozlov, M.V., Pollution impacts on bird population density and species diversity at four non-ferrous smelter sites, Biol. Conserv., 2012, vol. 150, no. 1, pp. 33–41.
Boch, S., Berlinger, M., Prati, D., and Fischer, M., Is fern endozoochory widespread among fern-eating herbivores?, Plant Ecol., 2016, vol. 217, no. 1, pp. 13–20.
Evstigneev, O.I., Voevodin, P.V., Korotkov, V.N., and Murashev, I.A., Zoochory and the distance of seed transfer in conifer–broadleaf forests of Eastern Europe, Usp. Sovrem. Biol., 2013, vol. 133, no. 4, pp. 392–400.
Berglund, Å.M.M. and Nyholm, N.E.I., Slow improvements of metal exposure, health- and breeding conditions of pied flycatchers (Ficedula hypoleuca) after decreased industrial heavy metal emissions, Sci. Tot. Environ., 2011, vol. 409, no. 20, pp. 4326–4334.
Eeva, T. and Lehikoinen, E., Long-term recovery of clutch size and egg shell quality of the pied flycatcher (Ficedula hypoleuca) in a metal polluted area, Environ. Pollut., 2015, vol. 201, pp. 26–33.
Chillo, V. and Anand, M., Effects of past pollution on zoochory in a recovering mixed temperate-boreal forest, Ecoscience, 2012, vol. 19, no. 3, pp. 258–265.
Belskaya, E., Gilev, A., and Belskii, E., Ant (Hymenoptera, Formicidae) diversity along a pollution gradient near the Middle Ural Copper Smelter, Russia, Environ. Sci. Pollut. Res., 2017, vol. 24, no. 11, pp. 10768–10777.
Garrido, J.L., Rey, P.J., and Herrera, C.M., Influence of elaiosome on postdispersal dynamics of an ant-dispersed plant, Acta Oecol., 2009, vol. 35, no. 3, pp. 393–399.
Lässig, R. and Močalov, S.A., Frequency and characteristics of severe storms in the Urals and their influence on the development, structure and management of the boreal forests, Forest Ecol. Manag., 2000, vol. 135, nos. 1–3, pp. 179–194.
Vorobeichik, E.L., Changes in the depth of forest litter under chemical pollution, Ekologiya, 1995, vol. 26, no. 4, pp. 278–284.
Xiong, S. and Nilsson, C., The effects of plant litter on vegetation: A meta-analysis, J. Ecol., 1999, vol. 87, no. 6, pp. 984–994.
Weltzin, J.F., Keller, J.K., Bridgham, S.D., et al., Litter controls plant community composition in a northern fen, Oikos, 2005, vol. 110, no. 3, pp. 537–546.
Verheyen, K., Guntenspergen, G.R., Biesbrouck, B., and Hermy, M., An integrated analysis of the effects of past land use on forest herb colonization at the landscape scale, J. Ecol., 2003, vol. 91, no. 5, pp. 731–742.
Vorobeichik, E.L., Trubina, M.R., Khantemirova, E.V., and Bergman, I.E., Long-term dynamic of forest vegetation after reduction of copper smelter emissions, Russ. J. Ecol., 2014, vol. 45, no. 6, pp. 498–507.
Rose, R., Monteith, D.T., Henrys, P., et al., Evidence for increases in vegetation species richness across UK environmental change network sites linked to changes in air pollution and weather patterns, Ecol. Indic., 2016, vol. 68, pp. 52–62.
Stevens, C.J., How long do ecosystems take to recover from atmospheric nitrogen deposition?, Biol. Conserv., 2016, vol. 200, Suppl. C, pp. 160–167.
ACKNOWLEDGMENTS
The author is grateful to E.L. Vorobeichik for providing data on metal concentrations in the litter, to I.N. Mikhailova for organizing field studies, and to the anonymous reviewer and E.L. Vorobeichik for their valuable comments on the manuscript.
Funding
This study was performed under state contract of the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Gorgolyuk
Supplementary material
Rights and permissions
About this article
Cite this article
Trubina, M.R. Vulnerability to Copper Smelter Emissions in Species of the Herb–Dwarf Shrub Layer: Role of Differences in the Type of Diaspore Dispersal. Russ J Ecol 51, 107–117 (2020). https://doi.org/10.1134/S1067413620020125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413620020125