Abstract
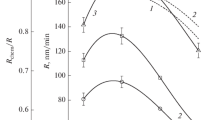
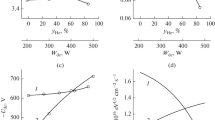
The influence of the type of active gas and the ratio of the concentration of the components of the CF4 + Ar, Cl2 + Ar, and HBr + Ar mixtures on the parameters of the plasma, steady-state concentrations of the active particles, and kinetics of reactive-ion etching of SiO2 is investigated. It is demonstrated that the rate of etching SiO2 decreases in the CF4–Cl2–HBr series and is characterized by nonmonotonic (with the maximum at 20–25% Ar) behaviour under the variation of the initial composition of the mixtures. It is found that the reasons for the nonmonotonicity for each of the mixtures are the increased effective probability of the interaction and decreased yield of etching due to the change in the physical parameters of the plasma and densities of the fluxes of the active particles.




Similar content being viewed by others
REFERENCES
Advanced Plasma Processing Technology, New York: Wiley, 2008.
Wolf, S. and Tauber, R.N., Silicon Processing for the VLSI Era, Vol. 1: Process Technology, New York: Lattice, 2000.
Nojiri, K., Dry Etching Technology for Semiconductors, Tokyo: Springer Int., 2015.
Rooth, J.R., Industrial Plasma Engineering, Philadelphia, IOP Publ., 2001.
Lieberman, M.A. and Lichtenberg, A.J., Principles of Plasma Discharges and Materials Processing, New York: Wiley, 2005.
Standaert, T.E.F.M., Hedlund, C., Joseph, E.A., and Oehrlein, G.S., Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide, J. Vac. Sci. Technol., A, 2004, vol. 22, pp. 53–60.
Gray, D.C., Tepermeister, I., and Sawin, H.H., Phenomenological modeling of ion enhanced surface kinetics in fluorine-based plasma etching, J. Vac. Technol., B, 1993, vol. 11, pp. 1243–1257.
Jin, W., Vitale, S.A., and Sawin, H.H., Plasma-surface kinetics and simulation of feature profile evolution in Cl2 + HBr etching of polysilicon, J. Vac. Sci. Technol., 2002, vol. 20, pp. 2106–2114.
Vitale, S.A., Chae, H., and Sawin, H.H., Silicon etching yields in F2, Cl2, Br2, and HBr high density plasmas, J. Vac. Sci. Technol., 2001, vol. 19, pp. 2197–2206.
Cheng, C.C., Guinn, K.V., Herman, I.P., and Donnelly, V.M., Competitive halogenation of silicon surfaces in HBr/Cl2 plasmas studied with X-ray photoelectron spectroscopy and in situ, realtime, pulsed laserinduced thermal desorption, J. Vac. Sci. Technol., A, 1995, vol. 13, pp. 1970–1976.
Ito, T., Karahashi, K., Kang, S.-Y., and Hamaguchi, S., Evaluation of Si etching yields by Cl+ Br+ and HBr+ ion irradiation, J. Phys.: Conf. Ser., 2010, vol. 232, p. 012021.
Lee, B.J., Efremov, A., Kim, J., Kim, C., and Kwon, K.-H., Peculiarities of Si and SiO2 etching kinetics in HBr + Cl2 + O2 inductively coupled plasma, Plasma Chem. Plasma Process., 2019, vol. 39, pp. 339–358.
Lee, B.J., Efremov, A., and Kwon, K.-H., Plasma parameters, gas-phase chemistry and Si/SiO2 etching mechanisms in HBr + Cl2 + O2 gas mixture: effect of HBr/O2 mixing ratio, Vacuum, 2019, vol. 163, pp. 110–118.
Efremov, A., Lee, J., and Kwon, K.-H., A comparative study of CF4, Cl2 and HBr + Ar inductively coupled plasmas for dry etching applications, Thin Solid Films, 2017, vol. 629, pp. 39–48.
Son, J., Efremov, A., Yun, S.J., Yeom, G.Y., and Kwon, K.-H., Etching characteristics and mechanism of SiNx films for nano-devices in CH2F2/O2/Ar inductively coupled plasma: effect of O2 mixing ratio, J. Nanosci. Nanotechnol., 2014, vol. 14, pp. 9534–9540.
Johnson, E.O. and Malter, L., A floating double probe method for measurements in gas discharges, Phys. Rev., 1950, vol. 80, no. 1, pp. 58–68.
Shun’ko, E.V., Langmuir Probe in Theory and Practice, Boca Raton, FL: Universal Publ., 2008.
Efremov, A., Lee, J., and Kim, J., On the control of plasma parameters and active species kinetics in CF4 + O2 + Ar gas mixture by CF4/O2 and O2/Ar mixing ratios, Plasma Chem. Plasma Process., 2017, vol. 37, pp. 1445–1462.
Efremov, A., Min, N.K., Choi, B.G., Baek, K.H., and Kwon, K.H., Model-based analysis of plasma parameters and active species kinetics in Cl2/X (X = Ar, He, N2) inductively coupled plasmas, J. Electrochem. Soc., 2008, vol. 155, no. 12, pp. D777–D782.
Kwon, K.H., Efremov, A., Kim, M., Min, N.K., Jeong, J., and Kim, K., A model-based analysis of plasma parameters and composition in HBr/X (X = Ar, He, N2) inductively coupled plasmas, J. Electrochem. Soc., 2010, vol. 157, no. 5, pp. H574–H579.
Lide, D.R., Handbook of Chemistry and Physics, New York, CRC, 1998–1999.
Efremov, A.M., Kim, D.P., and Kim, C.I., Simple model for ion-assisted etching using Cl2-Ar inductively coupled plasma: effect of gas mixing ratio, IEEE Trans. Plasma Sci., 2004, vol. 32, pp. 1344–1351.
Funding
This paper was supported by the Scientific Research Institute of System Analysis (SRISA/NIISI), Russian Academy of Sciences, project no. 0065-2019-0006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Levina
Abbreviation: EEDF—electron energy distribution function.
Rights and permissions
About this article
Cite this article
Efremov, A.M., Murin, D.B., Betelin, V.B. et al. Special Aspects of the Kinetics of Reactive Ion Etching of SiO2 in Fluorine-, Chlorine-, and Bromine-Containing Plasma. Russ Microelectron 49, 94–102 (2020). https://doi.org/10.1134/S1063739720010060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063739720010060