Abstract
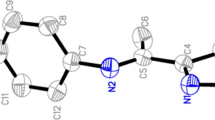
A simplified synthetic method was initiated to prepare the corresponding nickel complexes NiL2 (I–III) with direct condensation of mono(imino)pyrroles (L1–L3) and nickel dichloride, the structures and methyl methacrylate (MMA) catalytic polymerization behavior of this series of mono(imino)pyrrole nickel complexes were presented. The mono(imine)pyrrole ligands and the corresponding nickel complexes were determined by 1H NMR, 13C NMR, IR and MS, etc. Complexes I and III were further characterized by X-ray crystal diffraction (CIF files CCDC nos. 1890965 (I), 1890964 (III)). Both of the structures showed that the ligand chelated to nickel with 2 : 1 molar ratio. The systematic studies were focused on the relationship between the catalytic behavior of these complexes for MMA polymerization and catalyst structure, reaction time, reaction temperature, and ratio of monomer with catalyst. The optimum reaction conditions of the molar ratio of monomer to catalyst is of 1200 : 1, the polymerization temperature of 100°C, time of 10 h, the nickel complex with two bulky substituents on the o-position of phenyl ring linked with imine showed excellent catalytic activities for MMA polymerization (4.791 × 104 g mol–1 h–1), high molecular weight (Mn = 65.873 × 103 g mol–1), and narrow molecular mass distribution (polymer dispersity index = 3.9877), and azodiisobutyronitrile acted as co-catalyst during MMA polymerization.


Similar content being viewed by others
REFERENCES
Chen, W.Y., Lu, J., Li, J., et al., China Elastomerics, 2009, vol. 19, no. 4, p. 70.
Cui, X.M., Techno-Economics Petrochem., 2016, vol. 32, no. 4, p. 27.
Zhou, C.Y., Jiang, Y.W., and Xu, B., China Plastics Industry, 2011, vol. 39, no. 9, p. 5.
Yu, F.C., Inner Mongolia Petrochem.Industry, 2015, vol. 23, no. 24, p. 63.
Zhou, C.Y., Chem. Industry, 2015, vol. 33, no. 5, p. 41.
Fabri, F., Muteale, R.B., and De, O.W.A., Polymer, 2006, vol. 47, no. 13, p. 4544.
Yao, Y.M., Zhang, Y., Zhang, Z.Q., et al., Organometallics, 2003, vol. 22, no. 14, p. 2876.
You, S.M., Chatha, A.A., Jabbar, A., et al., Turk. J. Chem., 2007, vol. 31, no. 4, p. 471.
Ai, W., Tong, S.T., Mo, H.L., et al., J. Zhejiang Normal Univ., 2011, vol. 34, no. 2, p. 184.
Ding, L.Q., Chen, X.L., Li, M.G., et al., Xi’an Shiyou Univ. (Natural Sci. Ed.), 2018, vol. 33, no. 2, p. 82.
Johnson, L.M., Kilian, C.M., and Brookhart, M., J. Am. Chem. Soc., 1995, vol. 117, no. 23, p. 6414.
Killian, C.M., Tempel, D.J., Johnson, L.K., et al., J. Am. Chem. Soc., 1996, vol. 118, no. 46, p. 11664.
Kim, I., Hwang, J.M., Lee, J.K., et al., Macromol. Rapid Commun., 2003, vol. 24, no. 8, p. 508.
Wang, C.M., Friedrich, S., Younkin, T.R., et al., Organometallics, 1998, vol. 17, no. 15, p. 3149.
Bansleben, D.A., Friedrich, S.K., and Younkin, T.R., Transition Metal Complex Catalyst Compositions and Processes for Olefin Oligomerization and Polymerization: WO, 1998, p. 9842665.
Carlini, C., Martinelli, M., Galletti, A.M.R., et al., Macromol. Chem. Phys., 2002, vol. 203, no. 10, p. 1606.
Carlini, C., Martinelli, M., Gaiietiam, R., et al., Polym. Chem., 2003, vol. 41, no. 13, p. 2117.
Li, X.F., Li, Y.G., Li, Y.S., et al., Organometallics, 2005, vol. 24, no. 10, p. 2502.
Tang, G.R., and, Jin, G.X., Dalton Trans., 2007, vol. 34, no. 14, p. 3840.
Hu, Y.J., Jiang, H.L., and Wang, H.H., Industrial Catal., 2007, vol. 15, no. 6, p. 23.
Su, B.Y., Li, Y.N., Pan, D.D., et al., Current Organic Synthesis, 2019, vol. 16, no. 3, p. 444.
Su, B.Y., Wang, X.D., Wang, J.X., et al., J. Coord. Chem., 2015, vol. 68, no. 23, p. 4212.
Su, B.Y., Ta, H.B., and Zhang, Q.Z., Chin. J. Catal., 2011, vol. 32, no. 9, p. 1439.
Sheldrick, G.M., SADABS, Program for Bruker Area Detector Absorption Correction, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXS-97: Programs for Solution of Crystal Structure, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXL-97: Programs of Refinement for Crystal Structure, Göttingen: Univ. of Göttingen, 1997.
Yoshida, Y., Matsui, S., Takagi, Y., et al., Organometallics, 2004, vol. 20, no. 23, p. 4793.
Cuesta, L., Soler, T., and Urriolabeitia, E.P., Chem. Eur. J., 2012, vol. 18, no. 47, p. 15178.
Carabineiro, S.A., Silva, L.C., Gomes, P.T., et al., Inorg. Chem., 2007, vol. 46, no. 17, p. 6880.
Dawson, D.M., Walker, D.A., Thornton Pett, M., et al., Dalton Trans., 2000, vol. 41, no. 4, p. 459.
Orpen, A.G., Brammer, L., Allen, F.H., et al., J. Chem. Soc., Dalton Trans., 1989, vol. S1, no. 2, p. 83.
Su, B.Y., Li, X.T., Wang, J.X., et al., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, no. 12, p. 1053.
Anderson, C.E., Batsanov, A.S., Dyer, P.W., et al., Dalton Trans., 2006, vol. 45, no. 45, p. 5362.
Carabineiro, S.A., Silva, L.C., Gomes, P.T., et al., Inorg. Chem., 2007, vol. 46, no. 17, p. 6880.
Imhof, W. and Wunderle, J., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, vol. 68, p. o2741.
Imhof, W., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, vol. 69, no. 2, p. m96.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 51674200); the Science and Technology Research Program of Shaanxi Province (grant nos. 2018JM2035 and 2019JM-421).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, B.Y., Pan, D.D., Yan, T.Y. et al. Nickel(II) Complexes with Mono(imino)pyrrole Ligands: Preparation, Structure, and MMA Polymerization Behavior. Russ J Coord Chem 46, 355–364 (2020). https://doi.org/10.1134/S1070328420050073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328420050073