Abstract
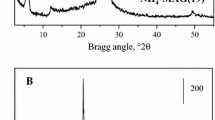
The samples of alumina of different origin exhibit different activity in the reaction of methanol dehydration to dimethyl ether (DME). To elucidate reasons for differences in their activity, a comparative study of the structural (XRD analysis, specific surface area, and porosity) and surface (Brønsted and Lewis acidity and hydroxyl coverage) properties of alumina samples was carried out. The presence of formates, aldehyde complexes, and bridging and terminal methoxy groups in different ratios on the surface of aluminum oxide samples under the reaction conditions of methanol conversion was detected using in situ IR spectroscopy. The reactivity of the detected surface compounds was compared for all of the samples. It was found that the activity of the samples in the formation of DME correlated with the rate of conversion of bridging methoxy groups. In turn, the rate of conversion of the bridging methoxy groups was determined by the acidity of bridging hydroxyl groups. An increase in acidity led to an increase in the bond strength of the methoxy group with the surface and a decrease in the rate of its conversion into DME. Linear methoxy groups underwent oxidative conversion into formate and aldehyde surface complexes. The nature of the oxidizing ability of an alumina surface is discussed.







Similar content being viewed by others
REFERENCES
Vishwanathan, M., Roh, H.-S., and Kim, J.-N., Catal. Lett., 2004, vol. 96, p. 23.
Tleimat-Manzalji, R., Bianchi, D., and Pajonk, G.M., React. Kinet. Catal. Lett., 1993, vol. 51, p. 29.
Bianchi, D., Chafik, T., Khalfallah, M., and Teichner, S.J., Appl. Catal., A, 1995, vol. 123, p. 89.
Sambeth, J.E., Juan, A., and Gambaro, L., J. Mol. Catal. A: Chem., 1997, vol. 118, p. 283.
Sambeth, J.E., Centeno, M.A., and Paul, A., J. Mol. Catal. A: Chem., 2000, vol. 161, p. 89.
Bakhtyari, A. and Rahimpour, M.R., Methanol. Science and Engineering. Ch. 10. Methanol to Dimethyl Ether, Amsterdam: Elsevier, 2018, p. 281.
Krylov, O.V. and Matyshak, V.A., Promezhutochnye soedineniya v geterogennom katalize (Intermediate Compounds in Heterogeneous Catalysis), Moscow: Nauka, 1996.
Matyshak, V.A., Khomenko, T.I., Lin, G.I., Zavalishin, I.N., and Rozovskii, A.Ya., Kinet. Catal., 1999, vol. 40, p. 269.
Neophytides, S.G., Marchi, A.J., and Froment, G.F., Appl. Catal., A, 1992, vol. 86, p. 45.
Martin, K.A. and Zabransky, R.F., Appl. Spectrosc., 1991, vol. 45, p. 68.
Davydov, A.A., IK-spektroskopiya v khimii poverkhnosti okislov (Infrared Spectroscopy in Chemistry of Oxides), Novosibirsk: Nauka, 1984.
Daturi, M., Binet, C., Lavalley, J-C., Galtayries, A., and Spoken, R., Phys. Chem. Chem. Phys., 1999, vol. 1, p. 5717.
Siokou, A. and Nix, R.M., J. Phys. Chem. B, 1999, vol. 103, p. 6984.
Novakova, J., Kubelkova, L., and Dolysek, Z., J. Catal., 1987, vol. 108, p. 208.
Lamotte, J., Moravek, V., Bensitel, M., and Lavalley, J.C., React. Kinet. Catal. Lett., 1988, vol. 36, p. 113.
Knop-Gericke, A., Havecker, M., Schedel-Niedrig, Th., and Schlogl, R., Top. Catal., 2000, vol. 10, p. 187.
Carley, A.P., Owens, A.W., Rajumon, M.K., Roberts, M.W., and Jackson, S.D., Catal. Lett., 1996, vol. 37, p. 79.
Ueno, A., Onishi, T., and Tamaru, K., Trans. Faraday Soc., 1971, vol. 67, p. 3585.
Matyshak, V.A., Berezina, L.A., Sil’chenkova, O.N., and Tret’yakov, V.F., Lin, G.I., Rozovskii A.Ya., Kinet. Catal., 2009, vol. 50, p. 111.
Pushkar, Y.N., Parenago, O.O., Fionov, A.V., and Lunina, E.V., Colloids Surf., A, 1999, vol. 158, p. 179.
Paukshtis, E.A., Infrakrasnaya spektroskopiya v geterogennom kislotno-osnovnom katalize (Infrared Spectroscopy in Heterogeneous Acid–Base Catalysis), Novosibirsk: Nauka, 1992.
Kipnis, M.A., Samokhin, P.V., Bondarenko, G.N., Volnina, E.A., Kostina, Yu.V., Yashina, O.V., Barabanov, V.G., and Kornilov, V.V., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 8, p. 1322.
Matyshak, V.A. and Krylov, O.V., Catal. Today, 1996, vol. 25, p. 1.
Malakhova, I.V., Ermolaev, V.K., and Danilova, I.G., Kinet. Catal., 2003, vol. 44, p. 536.
Alekseev, A.V., Lopatin, Yu.N., and Tsyganenko, A.A., React. Kinet. Catal. Lett., 1974, vol. 1, p. 443.
Busca, G., Rossi, P.F., and Lorenzelli, V., J. Phys. Chem., 1985, vol.89, p. 5433.
DeVito, D.A., Giardoni, F., and Kiwi-Minsker, L., J. Mol. Srtuct., 1999, vol. 469, p. 7.
Berezina, L.A., Matyshak, V.A., Korchak, V.N., Burdeinaya, T.N., Tret’yakov, V.F., Lin, G.I., and Rozovskii, A.Ya., Kinet. Catal., 2009, vol. 50, p. 775.
Matsushima, T. and White, J., J. Catal., 1976, vol. 44, p. 183.
Herd, A.C., Onishi, T., and Tamaru, K., Bull. Chem. Soc. Jpn., 1974, vol. 47, p. 575.
Yamashita, K., Naito, S., and Tamaru, K., J. Catal., 1985, vol. 94, p. 353.
Vishnetskaya, M.V., Taktarova, G.N., and Topchieva, K.V., Kinet. Katal., 1985, vol. 26, p. 1271.
Vishnetskaya, M.V., Taktarova, G.N., and Topchieva, K.V., Kinet. Katal., 1985, vol. 26, p. 1272.
Funding
This work was supported by the Federal Agency for Scientific Organizations (FANO) of Russia in accordance with a state contract (project no. V.46.13, 0082-2014-0007, state registration no. AAAA-A18-118020890105-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Abbreviations: DME, dimethyl ether; MF, methyl formate; TPD, thermally programmed desorption; EPR, electron paramagnetic resonance; BAS, Brønsted acid site; LAS, Lewis acid site.
Rights and permissions
About this article
Cite this article
Matyshak, V.A., Sadykov, V.A., Silchenkova, O.N. et al. Effect of the Surface Properties of γ-Al2O3 on the Conversion of Methanol into Dimethyl Ether According to in situ IR-Spectroscopic Data. Kinet Catal 61, 137–144 (2020). https://doi.org/10.1134/S0023158420010061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420010061