Abstract
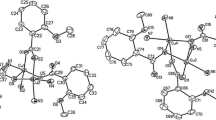
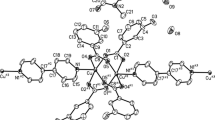
A dinuclear paddle-wheel Cu(II) complex [Cu2(L)4(H2O)2]·2H2O (1) [HL=2-(methoxycarbonyl)benzoic acid)] is crystallized and characterized by spectral and magnetic analyses and single crystal X-ray crystallography. Single crystal XRD reveals that the complex crystallizes in the triclinic P-1 space group and has a square pyramidal environment around each copper(II) atom with a Cu ··· Cu intramolecular distance of 2.631 Å. Noncovalent C-Hπp interactions generate a 1D chain, thus consolidating the crystal lattice. Such non-covalent interactions are verified theoretically by the Hirshfeld surface analysis, while a theoretical approach of molecular docking discloses the binding affinity of the complex with DNA which occurs interestingly in the major groove region through the free ester group present in the complex. Moreover, the temperature-variable magnetic data measured under an applied dc field of 0.1T in the temperature range 2-300 K show the presence of strong antiferromagnetic interactions between the two Cu(II) centres of the complex.
Similar content being viewed by others
References
B. Bleaney and K. D. Bowers. Proc. Roy. Soc. (Lond.) A, 1952, 214, 451.
O. Kahn. Molecular Magnetism. VCH: New York, 1993.
I. Chadjistamatis, A. Terzis, C. P. Raptopoulou, and S. P. Perlepes. Inorg. Chem. Comm., 2003, 6, 1365.
B. P. Baranwal, S. S. Das, and P. Singh. Synth. React. Inorg. Met. Org. Chem., 1998, 28, 1689.
E. J. Baran, S. B. Etcheverry, M. H. Torre, and E. Kremer. Polyhedron, 1994, 13, 1859.
G. C. Campbell and J. F. Haw. Inorg. Chem., 1998, 27, 3706.
M. Kasuni, K. G. Nik, P. Egedin, and A. Golobi. Acta Chim. Slov., 2010, 57, 350.
J. N. V. Niekerk and F. R. L. Schoening. Acta Cryst., 1953, 6, 227.
A. M. Qadir. J. Asian Chem., 2013, 25, 8829.
A. M. Thomas, M. Nethaji, S. Mahadevan, and A. R. Chakravarty. Inorg. Biochem., 2003, 94, 171.
L. Zhang, S. Y. Niu, J. Jin, L. P. Sun, G. D. Yang, and L. Yie. Chem. J. Chin. Universities, 2009, 30, 236.
G. Bullock, F. W. Hartstock, and L. Thomps. Can. J. Chem., 1983, 61, 57.
E. I. Solomon, U. M. Sundaram, and T. E. Machonkin. Chem. Rev., 1996, 96, 2563.
E. Borghi and P. L. Solari. J. Synchrotron Rad., 2005, 12, 102.
E. I. Solomon, K. W. Penfield, and D. E. Wilcox. Struct. Bond., 1983, 53, 1.
B. J. Hathaway. In: Comprehensive Coordination Chemistry / Eds. G. Wilkinson, R. D. Gillard, J. A. McCleverty. Pergamon Press: Oxford, 1987, 5(C53), 558.
W. Kaim and J. Rall. Angew. Chem. Int. Ed. Engl., 1996, 35, 43.
M. Melnik. Coord. Chem. Rev., 1982, 42, 259.
M. Kato and Y. Muto. Coord. Chem. Rev., 1988, 92, 45.
J. A. Ibers and W. C. Hamilton. International Tables for X-ray Crystallography. Kynoch Press: Birmingham, England, 1974, IV.
SMART&SAINT Software Reference manuals. Bruker Analytical X-ray Systems, Inc., Madison, WI, 2003, Version 6.45.
G. M. Sheldrick. SADABS, Software for Empirical Absorption Correction, Ver. 2.05, University of Gongen, Gongen, Germany, 2002.
XPREP, Version 5.1. Siemens Industrial Automation Inc., Madison, WI, 1995.
L. J. Bourhis, O. V. Dolomanov, R. J. Gildea, J. A. K. Howard, and H. Puschmann. Acta Crystallogr. Sect. A, 2015, 71, 59–71
A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and R. J. Spagna. J. Appl. Crystallogr., 1999, 32,115–119.
M. A. Spackman and D. Jayatilaka. CrystEngComm, 2009, 11, 19.
S. K. Seth, V. S. Lee, J. Yana, S. M. Zain, A. C. Cunha, V. F. Ferreira, A. K. Jordao, M. C. B. V. de Souza, S. M. S. V. Wardell, J. L. Wardellf, and E. R. T. Tiekink. CrystEngComm, 2015, 17, 2255.
S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka, and M. A. Spackman. CrystalExplorer, Version 3.0, University of Western Australia, 2012.
D. W. Ritche and V. Venkataraman. Bioinformatics, 2010, 26, 2398.
J. Lihui, L. Anchang, Z. Zongxi, and C. Yunfeng. J. Phys. Chem., 2011, 27, 1595.
Z. A. Siddiqi, M. Shahid, S. Kumar, M. Khalid, and S. Noor. J. Organomet. Chem., 2009, 694, 3768.
G. B. Deacon and R. J. Philips. Coord. Chem. Rev., 1980, 33, 227.
K. Nakamoto. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley-Interscience: New York, 1886, 191.
J. Lewis, and R. G. Wilkins. Modern Coordination Chemistry. Interscience Publisher: New York, 1960.
L. Sacconi. In: Transition Metal Chemistry / Ed. R. L. Carlin. Marcel Decker: New York, 1968, 221.
F. Sama, A. K. Dhara, M. N. Akhtar, Y.-C. Chen, M.-L. Tong, I. A. Ansari, M. Raizada, M. Ahmad, M. Shahid, and Z. A. Siddiqi. Dalton Trans., 2017, 46, 9801.
M. Raizada, F. Sama, M. Ashafaque, M. Shahid, M. Khalid, M. Ahmad, and Z. A. Siddiqi. Polyhedron, 2018, 139, 131.
S. K. Seth, D. Sarkar, and A. D. Jana, T. Kar. Cryst. Growth Des., 2011, 11, 4837.
R. Yadav, M. Trivedi, G.K. Kohn, R. Prasad, and A. Kumar. CrystEngComm, 2015, 17, 9175.
F. Sama, I. A. Ansari, M. Raizada, M. Ahmad, C. M. Nagaraja, M. Shahid, A. Kumar, K. Khan, and Z. A. Siddiqi. New J. Chem., 2017, 41, 1959.
I. A. Ansari, F. Sama, M. Raizada, M. Shahid, M. Ahmad, and Z. A. Siddiqi. New J. Chem., 2016, 40, 9840.
R. Rohs, I. Bloch, H. Sklenar, and Z. Shakked. Nucl. Acids Res., 2005, 33, 7048.
F. Arjmand, S. Parveen, M. Afzal, and M. Shahid. J. Photochem. Photobiol., 2012, 114, 15.
I. A. Ansari, F. Sama, M. Shahid, Rahisuddin, R. Arif, M. Khalid, and Z. A. Siddiqi. RSC Adv., 2016, 6, 11088.
R. Corradini, S. Sforza, T. Tedeshi, and R. Marchelli. Chirality, 2007, 19, 269.
P. Yang and M. Guo. Met.-Based Drugs, 1998, 5, 41.
M. N. Akhtar, Y.-C. Chen, M. A. A. Damen, and M.-L. Tong. Dalton Trans., 2017, 46, 116.
D. V. Yazigi, D. Aravena, E. Spodine, E. Ruiz, and S. Alvarez. Coord. Chem. Rev., 2010, 254, 2086.
Funding
Authors thank Chairperson, Department of Chemistry, Aligarh Muslim University, Aligarh for providing necessary research facilities and |DST-FIST, DST PURSE, and |UGC DRS-II (SAP) for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Vakil, F., Mantasha, I., Shahid, M. et al. A Dinuclear Paddle-Wheel Cu(II) Complex [Cu2(L)4(H2O)2]·2H2O [HL=2-(Methoxycarbonyl)Benzoic Acid)]: Crystallographic, Magnetic, and Theoretical Analyses. J Struct Chem 60, 1971–1982 (2019). https://doi.org/10.1134/S0022476619120138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476619120138