Abstract
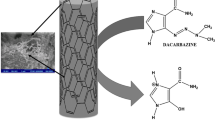
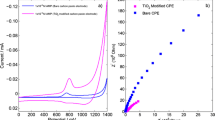
Electrochemical properties of regorafenib (REG) were studied in 0.1 M Britton−Robinson buffer-methanol solutions (3 : 2, v/v) with pH between 3 and 8 at carbon paste electrode by cyclic and differential pulse voltammetry. The results exhibited irreversible anodic peak at about 0.90 V vs. Ag/AgCl, NaCl (3 M). The anodic peak was found to be diffusion–adsorption controlled. Mechanism of REG electrochemical reaction was studied by performing density functional theory computations and mass spectrometric analysis. A validated differential pulse voltammetry (DPV) technique for REG determination was performed. The calibration curve of REG on carbon paste electrode was linear in the concentration range of 0.5–13 μg/mL and limit of detection was 0.10 µg/mL. The recommended DPV method was used to detect REG in spiked plasma and urine specimens and average recoveries were 94%.






Similar content being viewed by others
REFERENCES
Majithia, N. and Grothey, A., Expert Opin. Pharmacother., 2016, vol. 17, no. 1, p. 137.
Strumberg, D., Scheulen, M.E., Schultheis, B., Richly, H., Frost, A., Büchert, M., and Mross, K., Br. J. Cancer, 2012, vol. 106, no. 11, p. 1722.
Thangaraju, P., Singh, H., and Chakrabarti, A., Indian J. Cancer, 2015, vol. 52, no. 3, p. 257.
Prenen, H., Vecchione. L., and Van Cutsem, E., Target Oncol., 2013, vol. 8, no. 2, p. 83.
Rey, J.B., Launay-Vacher, V., and Tournigand, C., Target Oncol., 2015, vol. 10, no. 2, p. 199.
Zopf. D., Fichtner, I., Bhargava, A., Steinke, W., Thierauch, K.H., Diefenbach, K., Wilhelm, S., Hafner, F.T., and Gerisch, M., Cancer Med., 2016, vol. 5, no. 11, p. 3176.
Krishnamoorthy, S.K., Relias, V., Sebastian, S., Jayaraman, V., and Saif, M.W., Ther. Adv. Gastroenterol., 2015, vol. 8, no. 5, p. 285.
Fujita, K., Miura, M., and Shibata, H., Biomed. Chromatogr., 2016, vol. 30, no. 10, p. 1611.
Zhang, W.J., Li, Y., Wie, M.N., Chen, Y., Qiu, J.G., Jiang, Q.W., Yang, Y., Zheng, D.W., Qin, W.M., Huang, J.R., Wang, K., Zhang, W.J., Wang, Y.J., Yang, D.H., Chen, Z.S., and Shi, Z., Cancer Lett., 2017, vol. 386, no. 3, p. 100.
Romero, J.E., Chiva, J.A., Peris-Vicente, J., and Ochoa-Aranda, E., Bioanalysis, 2017, vol. 9, no. 9, p. 799.
Jayaprakash, R. and Natesan, S.K., J. Pharm. Chem., 2017, vol. 4, no. 1, p. 5.
Fei, Z., Yang, M., Shen, Y., Wang, Y., Qiu, F., and Lou, J., Lat. Am. J. Pharm., 2016, vol. 35, no. 6, p. 1415.
Ji, W., Zhang, Q., and Hu, L., Lat. Am. J. Pharm., 2014, vol. 33, no. 4, p. 607.
Van Dyk, M., Miners, J.O., Kichenadasse, G., McKinnon, R.A., and Rowland, A., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, vols. 1033–1034, p. 17.
Van Erp, N.P., de Wit, D., Guchelaar, H.J., Gelderblom, H., Hessing, T.J., and Den Hartigh, J., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, vol. 937, p. 33.
Hafner, F.T., Werner, D., and Kaiser, M., Bioanalysis, 2014, vol. 6, no. 14, p. 1923.
Luethi, D., Durmus, S., Schinkel, A.H., Schellens, J.H., Beijnen, J.H., and Sparidans, R.W., Biomed. Chromatogr., 2014, vol. 28, no. 10, p. 1366.
Allard, M., Khoudour, N., Rousseau, B., Joly, C., Costentin, C., Blanchet, B., Tournigand, C., and Hulin, A., J. Pharm. Biomed. Anal., 2017, vol. 142, p. 42.
Hu, X., Sun, M., Li, Y., and Tang, G., Colloids Surf., B, 2017, vol. 153, no. 5, p. 61.
Kauffmann, J.M., Patris, S., Vandeput, M., Sarakbi, A., and Sakira, A.K., Curr. Drug Delivery, 2016, vol. 13, no. 3, p. 371.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G, Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E., Jr., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J., Gaussian 09, Revision B.01, Wallingford: Gaussian, 2009.
Chai, J.D. and Head-Gordon, M., Phys. Chem. Chem. Phys., 2008, vol. 10, no. 44, p. 6615.
Barone, V., Cossi, M., and Tomasi, J., J. Chem. Phys., 1997, vol. 107, no. 8, p. 3210.
Cossi, M., Rega, N., Scalmani, G., and Barone, V., J. Comp. Chem., 2003, vol. 24, no. 6, p. 669.
ACKNOWLEDGMENTS
We wish to thank Bayer for supplying regorafenib and TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure) for the calculations reported in the theoretical part of this paper. We also thank to Uğur BOZKAYA for using Gaussian 09 program package.
Funding
This work was supported by Scientific Research Project Coordination Unit of Istanbul University, Project numbers: PYP-17194 and BEK-2017-25714.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Zeynep Aydoğmuş, Aslan, S.S., Yildiz, G. et al. Differential Pulse Voltammetric Determination of Anticancer Drug Regorafenib at a Carbon Paste Electrode: Electrochemical Study and Density Functional Theory Computations. J Anal Chem 75, 691–700 (2020). https://doi.org/10.1134/S1061934820050032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820050032