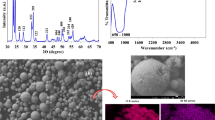
Abstract—
This paper presents a comparative analysis of the influence of acid and alkaline synthesis media on specific structural features of materials and their sorption characteristics during heat treatment of a precursor to calcium titanate. The liquid-phase synthesis medium is shown to influence the photocatalytic activity of CaTiO3 and its precursor.








Similar content being viewed by others
REFERENCES
Lewis, G., Properties of open-cell porous metals and alloys for orthopaedic applications, J. Mater. Sci. Mater. Med., 2013, vol. 24, no. 10, pp. 2293–2325. https://doi.org/10.1007/s10856-013-4998-y
Li, Y.H., Yang, C., Zhao, H.D., Qu, S.G., Li, X.Q., and Li, Y.Y., New developments of Ti-based alloys for biomedical applications, J. Mater., 2014, vol. 7, no. 3, pp. 1709–1800. https://doi.org/10.3390/ma7031709
Lei, X., Xu, B., Yang, G., Shi, T., Liu, D., and Yang, B., Direct calciothermic reduction of porous calcium titanate to porous titanium, J.Mater. Sci. Eng., C, 2018, vol. 91, pp. 125–134. https://doi.org/10.1016/j.msec.2018.05.027
Song, E., Chen, Y., Li, A., Sun, Y., Yang, R., Wang, J., Zhang, H., Li, J., and Zhang, D., Preparation and adsorbing performance of calcium titanate with template method, J. Mater. Manufact. Processes, 2017, vol. 32, no. 12, pp. 1428–1434. https://doi.org/10.1080/10426914.2017.1339320
Zhang, D. and Hou, P., Preparation of nano-calcium titanate powder and its adsorption behavior for lead ion and cadmium ion in water, J. Acta Chim. Sinica, 2009, vol. 67, pp. 1336–1342.
Zhang, D., Zhang, C.L., and Zhou, P., Preparation of porous nano-calcium titanate microspheres and its adsorption behavior for heavy metal ion in water, J. Hazard. Mater., 2011, vol. 186, nos. 2–3, pp. 971–977. https://doi.org/doi 10.1016/j.jhazmat.2010.11.096
Zhang, D., Wang, M., Ren, G.J., and Song, E.J., Preparation of biomorphic porous calcium titanate and its application for preconcentration of nickel in water and food samples, J.Mater. Sci. Eng., C, 2013, vol. 33, no. 8, pp. 4677–4683.https://doi.org/10.1016/j.msec.2013.07.030
Evans, I.R., Howard, J.A.K., Sreckovic, T., and Ristic, M.M., Variable temperature in situ X-ray diffraction study of mechanically activated synthesis of calcium titanate CaTiO3, J. Mater. Res. Bull., 2003, vol. 38, pp. 1203–1213. https://doi.org/10.1016/s0025-5408(03)00113-2
Kawashima, A., Matsubara, K., and Honda, K., Development of heterogeneous base catalysts for biodiesel production, J. Bioresour. Technol., 2008, vol. 99, no. 9, pp. 3439–3443. https://doi.org/10.1016/j.biortech.2007.08.009
Tyliszczak, B., Gaca, K.Z., Sobczak-Kupiec, A., and Dulian, P., Mechanochemical synthesis and investigations of calcium titanate powders and their acrylic dispersions, J. Eur. Ceram. Soc., 2014, vol. 34, no. 10, pp. 2259–2264. https://doi.org/10.1016/j.jeurceramsoc.2014.02.020
Kathirvel, K., Rajasekar, R., Kumar, S.A.R., and Sathiskumar, A., Effect of calcium titanium oxide coating on the power generation of solar cells, Int. J. Sci. Eng. Res., 2016, vol. 7, no. 4, pp. 127–130.
Khoei, N.A., Kharaziha, M., and Labbaf, S., Sol–gel synthesis of (Ca–Ba)TiO3 nanoparticles for bone tissue engineering, J. Ultrafine Grained Nanostruct. Mater., 2018, vol. 51, no. 1, pp. 77–83. https://doi.org/10.22059/JUFGNSM.2018.01.10
Souza, A.E., Silva, R.A., Santos, G.T.A., Moreira, M.L., Volanti, D.P., Teixeira, S.R., and Longo, E., Photoluminescence of barium–calcium titanates obtained by the microwave-assisted hydrothermal method (MAH), J. Chem. Phys. Lett., 2010, vol. 488, nos. 1–3, pp. 54–56. https://doi.org/10.1016/j.cplett.2010.01.065
Patil, B.M., Srinivasa, R.S., and Dharwadkar, S.R., Synthesis of CaTiO3 from calcium titanyl oxalate hexahydrate (CTO) as precursor employing microwave heating technique, J. Bull. Mater. Sci., 2007, vol. 30, no. 3, pp. 225–229. https://doi.org/10.1007/s12034-007-0040-7
Yahya, N.Y., Ngadi, N., Jusoh, M., and Halim, N.A.A., Characterization and parametric study of mesoporous calcium titanate catalyst for transesterification of waste cooking oil into biodiesel, J.Energy Conversion Management, 2016, vol. 129, pp. 275–283. https://doi.org/10.1016/j.enconman.2016.10.037
Yahya, N.Y., Ngadi, N., Wong, S., and Hassan, O., Transesterification of used cooking oil (UCO) catalyzed by mesoporous calcium titanate: kinetic and thermodynamic studies, J.Energy Conversion Management, 2018, vol. 164, pp. 210–218. https://doi.org/10.1016/j.enconman.2018.03.011
Yoshida, H., Zhang, L., Sato, M., Morikawa, T., Kajino, T., Sekito, T., Matsumoto, S., and Hirata, H., Calcium titanate photocatalyst prepared by a flux method for reduction of carbon dioxide with water, J. Catal. Today, 2015, vol. 251, pp. 132–139. https://doi.org/10.1016/j.cattod.2014.10.039
Korneev, N., Mayorga, D., Stepanov, S., Veenhuis, H., Buse, K., Kuper, C., Hesse, H., and Kratzig, E., Holographic and non-steady-state photocurrent characterization of photorefractive barium–calcium titanate, J. Opt. Commun., 1999, vol. 160, pp. 98–102. https://doi.org/10.1016/S0030-4018(98)00648-8
Ahmed, S., Rasul, M.G., Martens, W.N., Brown, R., and Hashib, M.A., Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review, J. Water,Air Soil Pollution, 2011, vol. 215, pp. 3–29. https://doi.org/10.1007/s11270-010-0456-3
Sakthivel, S., Janczarek, M., and Kisch, H., Visible light activity and photoelectrochemical properties of nitrogen doped TiO2, J. Phys. Chem. B, 2004, vol. 108, no. 50, pp. 19 384–19 387. https://doi.org/10.1021/jp046857q
Tripathy, N., Ghosh, S.P., and Kar, J.P., Transformation of sputtered calcium copper titanate thin film into nanorods by sequential annealing, J. Ceram. Int., 2018, vol. 44, no. 4, pp. 4052–4057. https://doi.org/10.1016/j.ceramint.2017.11.201
Redfern, S.A.T., High-temperature structural phase transitions in perovskite (CaTiO3), J. Phys.: Condens. Matter, 1996, vol. 8, pp. 8267–8275. https://doi.org/10.1088/0953-8984/8/43/019
Guyot, F., Richet, P., Courtial, P., and Gillet, P., High-temperature heat capacity and phase transitions of CaTiO3 perovskite, J. Phys. Chem. Miner., 1993, vol. 20, no. 3, pp. 141–146. https://doi.org/10.1007/bf00200116
Usinskas, P., Stankeviciute, Z., Beganskiene, A., and Kareiva, A., Sol–gel derived porous and hydrophilic calcium hydroxyapatite coating on modified titanium substrate, J. Surface Coat. Technol., 2016, vol. 307, pp. 935–940. https://doi.org/10.1016/j.surfcoat.2016.10.032
Ivanov, K.V., Agafonov, A.V., and Alekseeva, O.V., Mechanochemical synthesis and photocatalytic activity of calcium titanate, Izv. Vyssh. Uchebn. Zaved.,Ser.: Khim. Khim. Tekhnol., 2016, vol. 59, no. 6, pp. 83–89.
Agafonov, A.V., Ivanov, K.V., and Alekseeva, O.V., Low-temperature synthesis of barium titanate in aqueous solution, Izv. Vyssh. Uchebn. Zaved.,Ser.: Khim. Khim. Tekhnol., 2018, vol. 61, no. 12, pp. 56–62. https://doi.org/10.6060/ivkkt.20186112.5720
Ivanov, K.V. and Agafonov, A.V., Comparative parameters of the electrorheological effect in suspensions of nanosized barium titanyl acetates and titanyl oxalates in PMS-20 silicon oil, Prot. Met. Phys. Chem. Surf., 2014, vol. 50, no. 4, pp. 394–398. https://doi.org/10.1134/s2070205114040066
Ivanov, K.V., Alekseeva, O.V., Kraev, A.S., and Agafonov, A.V., Template-free synthesis and properties of mesoporous calcium titanate, Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 4, pp. 391–395. https://doi.org/10.1134/s2070205119040063
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., and Siemieniewska, T., Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem., 1985, vol. 57, no. 4, pp. 603–619. https://doi.org/10.1351/pac198557040603
Rouquerol, J., Avnir, D., Fairbridge, C.W., Everett, D.H., Haynes, J.M., Pernicone, N., Ramsay, J.D.F., Sing, K.S.W., and Unger, K.K., Recommendations for the characterization of porous solids (technical report), Pure Appl. Chem., 1994, vol. 66, no. 8, pp. 1739–1758. https://doi.org/10.1351/pac199466081739
Krzak-Ros, J., Filipiak, J., Pezowicz, C., Baszczuk, A., Baszczuk, A., Miller, M., Kowalski, M., and Będziński, R., The effect of substrate roughness on the surface structure o TiO2, SiO2, and doped thin films prepared by the sol–gel method, J. Acta Bioeng. Biomech., 2009, vol. 11, no. 2, pp. 21–29.
Ivanov, K.V., Agafonov, A.V., Baranchikov, A.E., Ivanov, V.K., Kozyukhin, S.A., Fatyushina, E.V., and Kozik, V.V., Influence of thermal treatment of nanometer-sized titanate and barium orthotitanate precursors on the electrorheological effect, J. Nanosyst.–Phys. Chem. Math., 2018, vol. 9, no. 6, pp. 746–753. https://doi.org/10.17586/2220-8054-2018-9-6-746-753
Hwang, U.Y., Park, H.S., and Koo, K.K., Behavior of barium acetate and titanium isopropoxide during the formation of crystalline barium titanate, J. Ind. Eng. Chem. Res., 2004, vol. 43, pp. 728–734. https://doi.org/10.1021/ie030276q
Xian, T., Yang, H., Dai, J.F., Wei, Z.Q., Ma, J.Y., and Feng, W.J., Photocatalytic properties of BiFeO3 nanoparticles with different sizes, J. Mater. Lett., 2011, vol. 65, pp. 1573–1575. https://doi.org/10.1016/j.matlet.2011.02.080
ACKNOWLEDGMENTS
We are grateful to our colleagues at the Verkhnevolzhsk Regional Physicochemical Characterization Center (Shared Research Facilities Center).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Ivanov, K.V., Alekseeva, O.V. & Agafonov, A.V. Photocatalytic Activity of the Products of Heat Treatment of Calcium Tetraacetate Titanyl and Calcium Tetrahydroxy Titanyl Prepared by Solution Techniques. Inorg Mater 56, 494–501 (2020). https://doi.org/10.1134/S0020168520040068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520040068