Abstract
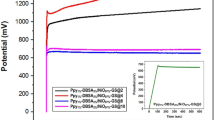
An electroactive material of a pseudocapacitor (PC) electrode based on highly dispersed MnO2 powders and its composites are obtained. The composition and surface morphology of these materials are investigated. The conditions for manufacturing electroactive paste from synthesized powders are determined. Technological methods are developed for the manufacture of the PC electrodes consisting of electroactive paste deposited on a conductive substrate (steel mesh). The electrochemical tests of the developed electrodes in an electrochemical cell are carried out using the method of cyclic voltammetry.



Similar content being viewed by others
Notes
The study was performed at the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
The study was performed at the Institute of Macromolecular Compounds, Russian Academy of Sciences.
The study was performed at the Engineering Center of the St. Petersburg State Technological Institute (Technical University).
REFERENCES
Zhang, L.L. and Zhao, X.S., Carbon-based materials as supercapacitor electrodes, Chem. Soc. Rev., 2009, vol. 38, pp. 2520–2531.
Yan, J., Sumboja, A., Wang, X., and Fu, C.P., Insights on the fundamental capacitive behavior: A case study of MnO2, Small, 2014, vol. 10, pp. 3568–3578.
Lv, P., Zhang, P., Feng, Y.Y., Li, Y., and Feng, W., High-performance electrochemical capacitors using electrodeposited MnO2 on carbon nanotube array grown on carbon fabric, Electrochim. Acta, 2012, vol. 78, pp. 515–523.
Zhu, G.Y., He, Z., Chen, J., Zhao, J., Feng, X.M., Yanwen, M., Quli, F., Lianhui, W., and Wei, H., Highly conductive three-dimensional MnO2–carbon nanotube–graphene–Ni hybrid foam as a binder-free supercapacitor electrode, Nanoscale, 2014, vol. 6, pp. 1079–1085.
Broughton, J.N. and Brett, M.J., Variations in MnO2 electrodeposition for electrochemical capacitors, Electrochim. Acta, 2005, vol. 50, pp. 4814–4819.
Ivanova, A.G., Zagrebel’nyi, O.A., Tsigas, A.A., and Shilova, O.A., Synthesis and electrophysical properties of the nanooxide layer of a pseudo-capacitor, Fiz. Khim. Stekla, 2012, vol. 38, no. 6, pp. 433–439.
Subramanian, V., Zhu, H.-Wei, and Wie, B., Nanostructured manganese oxides and their composites with carbon nanotubes as electrode materials for energy storage devices, Pure Appl. Chem., 2008, no. 11, pp. 2327–2343.
Tan, D.Z.W., Cheng, H., Nguyen, S.T., and Duong, H.M., Controlled synthesis of MnO2/CNT nanocomposites for supercapacitor applications, Mater. Technol., 2014, vol. 29, no. A2, pp. 107A–113A.
Wang, K., Gao, S., Du, Z., Yuan, A., Lu, W., and Chen, L., MnO2-carbon nanotube composite for high-areal-density supercapacitors with high rate performance, J. Power Sources, 2016, vol. 305, pp. 30–36.
Wang, J.-G., Yang, Y., Huang, Z.-H., and Kang, F., Synthesis and electrochemical performance of MnO2/CNTs-embedded carbon nanofibers nanocomposites for supercapacitors, Electrochim. Acta, 2012, vol. 75, pp. 213–219.
Daniel, B., Thierry, B., and Jerey, W.L., Manganese oxides: Battery materials make the leap to electrochemical capacitors, Electrochem. Soc. Interface, 2008, vol. 17, pp. 49–52.
Broughton, J.N. and Brett, M.J., Investigation of thin sputtered Mn films for electrochemical capacitors, Electrochim. Acta, 2004, vol. 49, pp. 4439–4446.
Yang, J., Lian, L., Ruan, H., Xie, F., and Wei, M., Nanostructured porous MnO2 on Ni foam substrate with a high mass loading via a CV electrodeposition route for supercapacitor application, Electrochim. Acta, 2014, vol. 136, pp. 189–194.
Reddy, R.N. and Reddy, R.G., Sol-gel MnO2 as an electrode material for electrochemical capacitors, J. Power Sources, 2003, vol. 124, no. 1, pp. 330–337.
Xu, M., Kong, L., Zhou, W., and Li, H., Hydrothermal synthesis and pseudocapacitance properties of α‑MnO2 hollow spheres and hollow urchins, J. Phys. Chem., 2007, vol. 111, no. 51, pp. 19141–19147.
Li, L., Hu, Z.A., An, N., Yang, Y.Y., Li, Z.M., and Wu, H.Y.J., Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability, Phys. Chem. C, 2014, vol. 118, no. 40, pp. 22865–22872.
Shi, K., Ren, M., and Zhitomirsky, I., Activated carbon-coated carbon nanotubes for energy storage in supercapacitors and capacitive water purification, ACS Sustainable Chem. Eng., 2014, vol. 2, pp. 1289–1298.
Feng, X., Yan, Z., Chen, N., Zhang, Y., Ma, Y., Liu, X., Fan, Q., Wang, L., and Huang, W., The synthesis of shape-controlled MnO2/graphene composites via a facile one-step hydrothermal method and their application in supercapacitors, J. Mater. Chem. A, 2013, vol. 1, pp. 12818–12825.
Zhao, Y., Ran, W., He, J., Huang, Y., Liu, Z., Liu, W., Tang, Y., Zhang, L., Gao, D., and Gao, F., High-performance asymmetric supercapacitors based on multilayer MnO2/graphene oxide nanoflakes and hierarchical porous carbon with enhanced cycling stability, Small, 2015, vol. 11, pp. 1310–1319.
Sychev, M.M., Kislotno-osnovnye kharakteristiki poverkhnosti tverdykh tel i upravlenie svoistvami materialov i kompozitov (Acid-Base Characteristics of the Surface of Solids and Control of the Properties of Materials and Composites), St. Petersburg: Khimizdat, 2016.
Nechiporenko, A.P., Donorno-aktseptornye svoistva poverkhnosti tverdofaznykh sistem. Indikatornyi metod (Donor-Acceptor Surface Properties of Solid-Phase Systems. Indicator Method), St. Petersburg: Lan’, 2017.
Hashem, A.M., Abuzeid, H.M., Abdel-Latif, A.M., Ehrenberg, H., Indris, S., Mauger, A., Groult, H., and Julien, C.M., MnO2 nano-rods prepared by redox reaction as cathodes in lithium batteries, ECS Trans., 2013, vol. 50, pp. 125–130.
Tucureanu, V., Matei, A., and Avram, A.M., FTIR spectroscopy for carbon family study, Crit. Rev. Anal. Chem., 2016, vol. 46, no. 6, pp. 502–520.
Ramirez-Castro, C., Crosnier, O., Athouёl, L., Retoux, R., Bélanger, D., and Brousse, T., Electrochemical performance of carbon/MnO2 nanocomposites prepared via molecular bridging as supercapacitor electrode materials, J. Electrochem. Soc., 2015, vol. 162, no. 5, pp. A5179–A5184.
Shin, J., Shin, D., Hwang, H., Yeo, T., Park, S., and Choi, W., One-step transformation of MnO2 into MnO2-x carbon nanostructures for high-performance supercapacitors using structure-guided combustion waves, J. Mater. Chem. A, 2017, vol. 5, pp. 13 488–13 498.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanova, A.G., Karasev, L.V., Masalovich, M.S. et al. Development and Research of Electroactive Pseudocapacitor Electrode Pastes Based on MnO2. Glass Phys Chem 46, 96–101 (2020). https://doi.org/10.1134/S1087659620010101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659620010101