Abstract
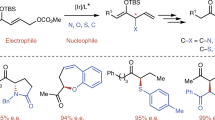
The catalytic asymmetric synthesis of novel and chiral amino acid derivatives with unconventional chemo-, diastereo-, and enantio-selectivity remains a paramount challenge. Herein we reported a novel protocol for the use of highly enantioselective copper-catalyzed cycloaddition of α,β-unsaturated acylsilanes as a springboard reaction for the facile synthesis of structurally diversified pyrrolidines and complicated α-amino esters by desilylation. The newly developed process could provide a wide range of synthetically useful acylsilane-substituted pyrrolidines (ASiP) in high yields and excellent diastereo- and enantio-selectivities with Cu/(R)-XylBINAP complex as the catalyst. And the downstream desilylation transformation enables to expand the potntial applications of 1,3-dipolar cycloaddition in the construction of structurally unique amino acid derivatives, in which an unprecedented and concerted fluoride anion-promoted C-X (X=H, Si, N, C) bond cleavage occurred to the enantioselective construction of aldehyde-substituted pyrrolidines, linear cinnamaldehyde or alkene-substituted amino esters in high ee values.
Similar content being viewed by others
References
Paquette LA. Science, 1982, 217: 793–800
Li L, Wei YL, Xu LW. Synlett, 2020, 31: 21–34
Reich HJ, Rusek JJ, Olson RE. J Am Chem Soc, 1979, 101: 2225–2227
Zhang HJ, Priebbenow DL, Bolm C. Chem Soc Rev, 2013, 42: 8540–8571
Schmink JR, Krska SW. J Am Chem Soc, 2011, 133: 19574–19577
Smirnov P, Mathew J, Nijs A, Katan E, Kami M, Bolm C, Apeloig Y, Marek I. Angew Chem Int Ed, 2013, 52: 13717–13721
Becker P, Pirwerdjan R, Bolm C. Angew Chem Int Ed, 2015, 54: 15493–15496
Becker P, Priebbenow DL, Pirwerdjan R, Bolm C. Angew Chem Int Ed, 2014, 53: 269–271
Gonzalez J, Santamaria J, Ballesteros A. Angew Chem Int Ed, 2015, 54: 13678–13681
Capaldo L, Riccardi R, Ravelli D, Fagnoni M. ACS Catal, 2018, 8: 304–309
Ito K, Tamashima H, Iwasawa N, Kusama H. J Am Chem Soc, 2011, 133: 3716–3719
Zhang FG, Eppe G, Marek I. Angew Chem Int Ed, 2016, 55: 714–718
Tugny C, Zhang FG, Marek I. Chem Eur J, 2019, 25: 205–209
Feng JJ, Oestreich M. Angew Chem Int Ed, 2019, 58: 8211–8215
Sasaki M, Oyamada K, Takeda K. J Org Chem, 2010, 75: 3941–3943
Takeda K, Nakajima A, Takeda M, Okamoto Y, Sato T, Yoshii E, Koizumi T, Shiro M. J Am Chem Soc, 1998, 120: 4947–4959
Cao W, Tan D, Lee R, Tan CH. J Am Chem Soc, 2018, 140: 1952–1955
Arai N, Suzuki K, Sugizaki S, Sorimachi H, Ohkuma T. Angew Chem Int Ed, 2008, 47: 1770–1773
Rong J, Oost R, Desmarchelier A, Minnaard AJ, Harutyunyan SR. Angew Chem Int Ed, 2015, 54: 3038–3042
Leibeling M, Shurrush KA, Werner V, Perrin L, Marek I. Angew Chem Int Ed, 2016, 55: 6057–6061
Nagy A, Collard L, Indukuri K, Leyssens T, Riant O. Chem Eur J, 2019, 25: chem.201901785
Maruoka K, Imoto H, Yamamoto H. J Am Chem Soc, 1994, 116: 12115–12116
Takeda K, Sawada Y, Sumi K. Org Lett, 2012, 4: 1031–1033
Bi J, Ma R, Yang J. Chin J Org Chem, 2018, 38: 2553–2570
Patel N, Sood R, Bharatam PV. Chem Rev, 2018, 118: 8770–8785
Yang J, Zhao J. Sci China Chem, 2018, 61: 97–112
Chen X, Luo W, Ma H, Peng Q, Yuan WZ, Zhang Y. Sci China Chem, 2018, 61: 351–359
Näjera C, Sansano JM. Pure Appl Chem, 2019, 91: 575–596
Pellissier H. Chem Rev, 2016, 116: 14868–14917
Wei L, Xiao L, Hu Y, Wang Z, Tao H, Wang C. Chin J Org Chem, 2019, 39: 2119–2130
Bai XF, Song T, Xu Z, Xia CG, Huang WS, Xu LW. Angew Chem Int Ed, 2015, 54: 5255–5259
Bai XF, Xu Z, Xia CG, Zheng ZJ, Xu LW. ACS Catal, 2015, 5: 6016–6020
Zhao Q, Vuong TMH, Bai XF, Pannecoucke X, Xu LW, Bouillon JP, Jubault P. Chem Eur J, 2018, 24: 5644–5651
Yu B, Yang KF, Bai XF, Cao J, Zheng ZJ, Cui YM, Xu Z, Li L, Xu LW. Org Lett, 2018, 20: 2551–2554
Yuan Y, Zheng ZJ, Li L, Bai XF, Xu Z, Cui YM, Cao J, Yang KF, Xu LW. Adv Synth Catal, 2018, 360: 3002–3008
Yuan Y, Zheng ZJ, Ye F, Ma JH, Xu Z, Bai XF, Li L, Xu LW. Org Chem Front, 2018, 5: 2759–2764
Stergiades IA, Tius MA. J Org Chem, 1999, 64: 7547–7551
Nikolaev A, Orellana A. Org Lett, 2015, 17: 5796–5799
Degl’Innocenti A, Pike S, Walton DRM. Chem Commun, 1980, 24: 1201–1202
Capperucci A, Degl’Innocenti A, Dondoli P, Nocentini T, Reginato G, Ricci A. Tetrahedron, 2001, 57: 6267–6276
Mattson AE, Bharadwaj AR, Scheidt KA. J Am Chem Soc, 2004, 126: 2314–2315
Nicewicz DA, Satterfield AD, Schmitt DC, Johnson JS. J Am Chem Soc, 2008, 130: 17281–17283
Liang T, Zhao H, Gong L, Jiang H, Zhang M. iScience, 2019, 15: 127–135
Bai XF, Deng WH, Xu Z, Li FW, Deng Y, Xia CG, Xu LW. Chem Asian J, 2014, 9: 1108–1115
Cai YF, Li L, Luo MX, Yang KF, Lai GQ, Jiang JX, Xu LW. Chirality, 2011, 23: 397–403
Wang CY, Qin ZY, Huang YL, Jin RX, Lan Q, Wang XS. iScience, 2019, 21: 490–498
Kong L, Biletskyi B, Nuel D, Clavier H. Org Chem Front, 2018, 5: 1600–1603
Decostanzi M, Godemert J, Oudeyer S, Levacher V, Campagne JM, Leclerc E. Adv Synth Catal, 2016, 358: 526–531
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21773051, 21702211, 21703051), Zhejiang Provincial Natural Science Foundation of China (LZ18B020001, LQ19B040001, LY18B020013), and the Hangzhou Science and Technology Bureau of China (20180432B05). The authors thank K.Z. Jiang and X.Q. Xiao for their assistance on the MS and X-ray analysis, the technicians of our group, and the members of our NMR, MS and HPLC departments for their excellent service. L.-W. Xu also thank Prof. X.Q. Hu (Zhejiang University of Technology) and Prof. Y. Lu (National University of Singapore) for their help and discussion in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ma, JH., Li, L., Sun, YL. et al. Silicon-mediated enantioselective synthesis of structurally diverse α-amino acid derivatives. Sci. China Chem. 63, 1082–1090 (2020). https://doi.org/10.1007/s11426-020-9768-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9768-x