Abstract
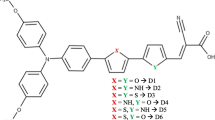
In this work, a series of six organic dyes-sensitized solar cells (DSSCs) combining various π-bridges with a fixed donor (triphenylamine) and a fixed electron acceptor (cyanoacrylic acid), namely D1-6, were studied. The geometrical structure, electronic and optical properties of these dyes have been investigated with the density functional theory and TD-BHandHLYP hybrid functional (time-dependent Becke-Half-and-Half-Lee–Yang–Parr’s) methods. The effects of π-bridging of the dyes have shown that the rings with a sulfur atom reduce the energy gaps and provide a redshift of the absorption spectra. Similarly, we focus on the description of the ground and excited state properties. On the other hand, the pyrrole group improves the open-circuit voltage (VOC) and the light-harvesting efficiency parameters leading to greater power conversion efficiency. Furthermore, the results revealed lowest total reorganization energy λ (λ+ and λ−) for the dye D3 with pyrrole linkage, which reflects its most favorable charge-transport properties, implying a lower charge recombination rate, faster charge injection and dye regeneration processes. Therefore, this study would provide a new path to design novel conjugated organic molecules as dyes for high-performance DSSCs.





Similar content being viewed by others
References
B. O’Regan, M. Grätzel, Nature 353, 737 (1991)
J.-X. Cheng, Z.-S. Huang, L. Wang, D. Cao, Dyes Pigm. 131, 134 (2016)
Y. Wu, W. Zhu, Chem. Soc. Rev. 42, 2039 (2013)
X. Liu, Y. Luo, H. Li, Y. Fan, Z. Yu, Y. Lin, L. Chen, Q. Meng, Chem. Commun 0, 2847 (2007)
J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan, G. Luo, Chem. Rev. 115, 2136 (2015)
J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan, G. Luo, Y. Lin, Y. Xie, Y. Wei, Chem. Soc. Rev. 46, 5975 (2017)
A. Mahmood, Sol. Energy 123, 127 (2016)
A. Carella, F. Borbone, R. Centore, Front. Chem. 6, 481 (2018)
M. Liang, W. Xu, F. Cai, P. Chen, B. Peng, J. Chen, Z. Li, J. Phys. Chem. C 111, 4465 (2007)
R. Li, X. Lv, D. Shi, D. Zhou, Y. Cheng, G. Zhang, P. Wang, J. Phys. Chem. C 113, 7469 (2009)
N.P. Liyanage, A. Yella, M. Nazeeruddin, M. Grätzel, J.H. Delcamp, A.C.S. Appl, Mater. Interfaces 8, 5376 (2016)
S. Ennehary, H. Toufik, S. M. Bouzzine, and F. Lamchouri, J. Comput. Electron. (2020).
Y.K. Eom, J.Y. Hong, J. Kim, H.K. Kim, Dyes Pigm. 136, 496 (2017)
A. Irfan, A.G. Al-Sehemi, S. Muhammad, M.S. Al-Assiri, A.R. Chaudhry, A. Kalam, M. Shkir, J. King Saud Univ. Sci. 27, 361 (2015)
L.L. Estrella, S.H. Lee, D.H. Kim, Dyes Pigm. 165, 1 (2019)
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
R. Krishnan, J.S. Binkley, R. Seeger, J.A. Pople, J. Chem. Phys. 72, 650 (1980)
Z.M.E. Fahim, S.M. Bouzzine, A.A. Youssef, M. Bouachrine, M. Hamidi, Comput. Theoret. Chem. 1125, 39 (2018)
V. Barone, M. Cossi, J. Phys. Chem. A 102, 1995 (1998)
T. Yanai, D.P. Tew, N.C. Handy, Chem. Phys. Lett. 393, 51 (2004)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996)
A. Üngördü, Chem. Phys. Lett. 733, 136696 (2019)
M.P. Balanay, D.H. Kim, J. Mol. Struct. (Thoechem) 910, 20 (2009)
W.-L. Ding, D.-M. Wang, Z.-Y. Geng, X.-L. Zhao, W.-B. Xu, Dyes Pigm. 98, 125 (2013)
Gaussian 09, R.A.: 1, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, v. Barone, b. Mennucci, ga petersson et al., gaussian. Inc Wallingford CT. 121, 150 (2009).
A. Aicha Youssef, S. Mohamed Bouzzine, Z. Mohyi Eddine Fahim, İ. Sıdır, M. Hamidi, M. Bouachrine, Phys. B Condensed Matter 560, 111 (2019)
H. Toufik, S. M. Bouzzine, O. Ninis, M. Aberkane, F. Lamchouri, M. Hamidi, and M. Bouachrine, Жypнaл Фiзичниx Дocлiджeнь 1702 (2012).
H. Toufik, S.M. Bouzzine, O. Ninis, F. Lamchouri, M. Aberkane, M. Hamidi, M. Bouachrine, Res. Chem. Intermed. 38, 1375 (2012)
M. Lazrak, H. Toufik, S.M. Bouzzine, H. Bih, F. Lamchouri, IOP Conf. Ser. Earth Environ. Sci. 161, 012021 (2018)
M. Lazrak, H. Toufik, S. M. Bouzzine, H. Bih, and F. Lamchouri, 10 (n.d.).
J.H. Bae, S.J. Lim, J. Choi, S.B. Yuk, J.W. Namgoong, J.H. Ko, W. Lee, J.P. Kim, Dyes Pigm. 162, 905 (2019)
C. Figueira, P. Lopes, C. Gomes, L. F. Veiros, P. Gomes, Exploring the influence of steric hindrance and electronic nature of substituents in the supramolecular arrangements of 5-(Substituted Phenyl)-2-Formylpyrroles (2015).
Thin Film Physics Division, Department of Physics, Chemistry, and Biology (IFM), Linköping University, Sweden and S. Khromov, Doping Effects on the Structural and Optical Properties of GaN (Linköping University Electronic Press, Linköping, 2013).
J.B. Asbury, Y.-Q. Wang, E. Hao, H.N. Ghosh, T. Lian, Res Chem Intermediat 27, 393 (2001)
A. Hagfeldt, M. Graetzel, Chem. Rev. 95, 49 (1995)
L.L. Estrella, M.P. Balanay, D.H. Kim, J. Phys. Chem. A 120, 5917 (2016)
J.C. Delgado, Y. Ishikawa, R.G. Selsby, Photochem. Photobiol. 85, 1286 (2009)
W.R. Duncan, O.V. Prezhdo, Annu. Rev. Phys. Chem. 58, 143 (2007)
B.C. Lin, C.P. Cheng, Z.P.M. Lao, J. Phys. Chem. A 107, 5241 (2003)
G.R. Hutchison, M.A. Ratner, T.J. Marks, J. Am. Chem. Soc. 127, 2339 (2005)
H. Li, L. Yang, R. Tang, Y. Hou, Y. Yang, H. Wang, H. Han, J. Qin, Q. Li, Z. Li, Dyes Pigm. 99, 863 (2013)
R. Manne, T. Åberg, Chem. Phys. Lett. 7, 282 (1970)
J. Preat, C. Michaux, D. Jacquemin, E.A. Perpète, J. Phys. Chem. C 113, 16821 (2009)
Z.M.E. Fahim, S.M. Bouzzine, Y. Ait Aicha, M. Bouachrine, M. Hamidi, Res. Chem. Intermed. 44, 2009 (2018)
K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara, H. Arakawa, J. Phys. Chem. B 107, 597 (2003)
W. Sang-aroon, S. Laopha, P. Chaiamornnugool, S. Tontapha, S. Saekow, V. Amornkitbamrung, J Mol Model 19, 1407 (2013)
A. SalimiBeni, M. Zarandi, B. Hosseinzadeh, A. NajafiChermahini, J. Mol. Struct. 1164, 155 (2018)
L.-Y. Lin, C.-H. Tsai, K.-T. Wong, T.-W. Huang, L. Hsieh, S.-H. Liu, H.-W. Lin, C.-C. Wu, S.-H. Chou, S.-H. Chen, A.-I. Tsai, J. Org. Chem. 75, 4778 (2010)
Acknowledgements
This work was realized with the support of the National Center for Scientific and Technical Research (CNRST—Morocco) as part of the Research Excellence Awards Program (No. 28USMBA2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lazrak, M., Toufik, H., Bouzzine, S.M. et al. Bridge effect on the charge transfer and optoelectronic properties of triphenylamine-based organic dye sensitized solar cells: theoretical approach. Res Chem Intermed 46, 3961–3978 (2020). https://doi.org/10.1007/s11164-020-04184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04184-x