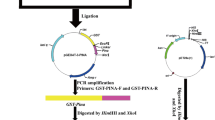
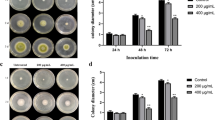
Abstract
Aspergillus flavus infection is a major issue for safe food storage. In this study, we constructed an efficient prokaryotic expression system for puroindoline B (PINB) protein to detect its antifungal activity. The Puroindoline b gene was cloned into pET-28a (+) vector and expressed in Escherichia coli. Treatment with fusion PINB revealed that it inhibits mycelial growth of A. flavus, a common grain mold. Moreover, fusion PINB-treated A. flavus mycelium withered and exhibited a sunken spore head. As fusion PINB concentration increased, electrical conductivity in mycelium also increased, indicative of cell membrane damage. Furthermore, intracellular malate dehydrogenase and succinate dehydrogenase activity decreased, revealing a disruption in the tricarboxylic acid cycle. Moreover, the dampened activity of the ion pump Na+K+-ATPase negatively affected the intracellular regulation of both ions. Catalase and superoxide dismutase activity decreased, thus reducing antioxidant capacity, a result confirmed with an increase in malondialdehyde content. Changes to the GSH/GSSG ratio indicated a shift to an intracellular oxidative state. At the same time, laser scanning confocal microscopy assay showed the accumulation of reactive oxygen species and nuclear damage. Therefore, the PINB fusion protein may have the potential to control A. flavus in grain storage and food preservation.







Similar content being viewed by others
References
Lahouar A, Marin S, Crespo-Sempere A, Saïd S, Sanchis V (2016) Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic Aspergillus flavus isolates on sorghum seeds. Rev Argent Microbiol 48(1):78–85. https://doi.org/10.1016/j.ram.2015.10.001
Astoreca A, Vaamonde G, Dalcero A, Marin S, Ramos A (2014) Abiotic factors and their interactions influence on the co-production of aflatoxin B1 and cyclopiazonic acid by Aspergillus flavus isolated from corn. Food Microbiol 38:276–283. https://doi.org/10.1016/j.fm.2013.07.012
Masiello M, Somma S, Ghionna V, Logrieco AF, Moretti A (2019) In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 11(1):18. https://doi.org/10.3390/toxins11010011
Casquete R, Benito MJ, Córdoba MG, Ruiz-Moyano S, Martín A (2017) The growth and aflatoxin production of Aspergillus flavus strains on a cheese model system are influenced by physicochemical factors. J Dairy Sci 100(9):6987–6996. https://doi.org/10.3168/jds.2017-12865
Prencipe S, Siciliano I, Contessa C, Botta R, Garibaldi A, Gullino ML, Spadaro D (2018) Characterization of Aspergillus section Flavi isolated from fresh chestnuts and along the chestnut flour process. Food Microbiol 69:159–169. https://doi.org/10.1016/j.fm.2017.08.004
Al-Dhabi NA, Valan Arasu M (2018) Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials 8(7):500. https://doi.org/10.3390/nano8070500
Kimura H, Asano R, Tsukamoto N, Tsugawa W, Sode K (2018) Convenient and universal fabrication method for antibody–enzyme complexes as sensing elements using the SpyCatcher/SpyTag system. Anal Chem 90(24):14500–14506. https://doi.org/10.1021/acs.analchem.8b04344
Abdel-Kareem MM, Rasmey AM, Zohri AA (2019) The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Lett Appl Microbiol 68(2):104–111. https://doi.org/10.1111/lam.13105
Chaudhari AK, Singh VK, Dwivedy AK, Das S, Upadhyay N, Singh A, Dkhar MS, Kayang H, Prakash B, Dubey NK (2020) Chemically characterised Pimenta dioica (L.) Merr. essential oil as a novel plant based antimicrobial against fungal and aflatoxin B1 contamination of stored maize and its possible mode of action. Nat Prod Res 34(5):745–749. https://doi.org/10.1080/14786419.2018.1499634
Devipriya D, Roopan SM (2019) UV-light intervened synthesis of imidazo fused quinazoline and its solvatochromism, antioxidant, antifungal and luminescence properties. J Photochem Photobiol B 190:42–49. https://doi.org/10.1016/j.jphotobiol.2018.11.003
Kim JE, Oh YJ, Song AY, Min SC (2019) Preservation of red pepper flakes using microwave-combined cold plasma treatment. J Sci Food Agric 99(4):1577–1585. https://doi.org/10.1002/jsfa.9336
Hu Y, Zhang J, Kong W, Zhao G, Yang M (2017) Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem 220:1–8. https://doi.org/10.1016/j.foodchem.2016.09.179
Yadav SKR, Sahu T, Dixit A (2016) Structural and functional characterization of recombinant napin-like protein of momordica charantia expressed in methylotrophic yeast Pichia pastoris. Appl Microbiol Biotechnol 100(15):6703–6713. https://doi.org/10.1007/s00253-016-7446-3
Yu Y, Zhang G, Li Z, Cheng Y, Gao C, Zeng L, Chen J, Yan L, Sun X, Guo L, Yan Z (2017) Molecular cloning, recombinant expression and antifungal activity of BnCPI, a Cystatin in Ramie (Boehmeria nivea L.). Genes 8(10):265. https://doi.org/10.3390/genes8100265
Bogdanov IV, Shenkarev ZO, Finkina EI, Melnikova DN, Rumynskiy EI, Arseniev AS, Ovchinnikova TV (2016) A novel lipid transfer protein from the pea Pisum sativum: isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic properties. BMC Plant Biol 16(1):107. https://doi.org/10.1186/s12870-016-0792-6
Liang Y, Kong Q, Yao Y, Xu S, Xie X (2019) Fusion expression and anti-Aspergillus flavus activity of a novel inhibitory protein DN-AflR. Int J Food Microbiol 290:184–192. https://doi.org/10.1016/j.ijfoodmicro.2018.10.015
Ishida H, Nguyen LT, Gopal R, Aizawa T, Vogel HJ (2016) Overexpression of antimicrobial, anticancer, and transmembrane peptides in Escherichia coli through a calmodulin-peptide fusion system. J Am Chem Soc 138(35):11318–11326. https://doi.org/10.1021/jacs.6b06781
Blochet JE, Chevalier C, Forest E, Pebay-Peyroula E, Gautier MF, Joudrier P, Pézolet M, Marion D (1993) Complete amino acid sequence of puroindoline, a new basic and cystine-rich protein with a unique tryptophan-rich domain, isolated from wheat endosperm by Triton X-114 phase partitioning. FEBS Lett 329(3):336–340. https://doi.org/10.1016/0014-5793(93)80249-T
Haney EF, Petersen AP, Lau CK, Jing W, Storey DG, Vogel HJ (2013) Mechanism of action of puroindoline derived tryptophan-rich antimicrobial peptides. Biochim Biophys Acta 1828(8):1802–1813. https://doi.org/10.1016/j.bbamem.2013.03.023
Sanders MR, Clifton LA, Frazier RA, Green RJ (2017) Tryptophan to arginine substitution in puroindoline-b alters binding to model eukaryotic membrane. Langmuir 33(19):4847–4853. https://doi.org/10.1021/acs.langmuir.6b03030
Alfred RL, Palombo EA, Panozzo JF, Bhave M (2013) The antimicrobial domains of wheat puroindolines are cell-penetrating peptides with possible intracellular mechanisms of action. PLoS One 8(10):e75488. https://doi.org/10.1371/journal.pone.0075488
Miao Y, Chen L, Wang C, Wang Y, Zheng Q, Gao C, Yang G, He G (2012) Expression, purification and antimicrobial activity of puroindoline a protein and its mutants. Amino Acids 43(4):1689–1696. https://doi.org/10.1007/s00726-012-1250-x
Dubreil L, Gaborit T, Bouchet B, Gallant DJ, Broekaert WF, Quillien L, Marion D (1998) Spatial and temporal distribution of the major isoforms of puroindolines (puroindoline-a and puroindoline-b) and non specific lipid transfer protein (ns-LTP1e1) of Triticum aestivum seeds: relationships with their in vitro antifungal properties. Plant Sci 138(2):121–135. https://doi.org/10.1016/S0168-9452(98)00121-6
Capparelli R, Amoroso MG, Palumbo D, Iannaccone M, Faleri C, Cresti M (2005) Two plant puroindolines colocalize in wheat seed and in vitro synergistically fight against pathogens. Plant Mol Biol 58(6):857–867. https://doi.org/10.1007/s11103-005-8270-9
Shagaghi N, Alfred RL, Clayton AHA, Palombo EA, Bhave M (2016) Anti-biofilm and sporicidal activity of peptides based on wheat puroindoline and barley hordoindoline proteins. J Pept Sci 22(7):492–500. https://doi.org/10.1002/psc.2895
Capparelli R, Ventimiglia I, Palumbo D, Nicodemo D, Salvatore P, Amoroso MG, Iannaccone M (2007) Expression of recombinant puroindolines for the treatment of staphylococcal skin infections (acne vulgaris). J Biotechnol 128(3):606–614. https://doi.org/10.1016/j.jbiotec.2006.11.004
Capparelli R, Palumbo D, Iannaccone M, Ventimiglia I, Di Salle E, Capuano F, Salvatore P, Amoroso MG (2006) Cloning and expression of two plant proteins: similar antimicrobial activity of native and recombinant form. Biotechnol Lett 28(13):943–949. https://doi.org/10.1007/s10529-006-9031-9
Caporale C, Di Berardino I, Leonardi L, Bertini L, Cascone A, Buonocore V, Caruso C (2004) Wheat pathogenesis-related proteins of class 4 have ribonuclease activity. FEBS Lett 575:71–76. https://doi.org/10.1016/j.febslet.2004.07.091
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22(11):1399–1408. https://doi.org/10.1038/nbt1029
Maxwell KL, Bona D, Liu C, Arrowsmith CH, Edwards AM (2003) Refolding out of guanidine hydrochloride is an effective approach for high-throughput structural studies of small proteins. Protein Sci 12(9):2073–2080. https://doi.org/10.1110/ps.0393503
Clifton LA, Green RJ, Frazier RA (2007) Puroindoline-b mutations control the lipid binding interactions in mixed puroindoline-a:puroindoline-b systems. Biochemistry 46(48):13929–13937. https://doi.org/10.1021/bi701680w
Wang QY, Lin QL, Peng K, Cao JZ, Yang C, Xu D (2017) Surfactin variants from Bacillus subtilis natto CSUF5 and their antifungal properities against Aspergillus niger. J Biobased Mater Bioenergy 11(3):210–215. https://doi.org/10.1166/jbmb.2017.1665
Moon TM, D'Andréa ÉD, Lee CW, Soares A, Jakoncic J, Desbonnet C, Garcia-Solache M, Rice LB, Page R, Peti W (2018) The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into β-lactam resistance. J Biol Chem 293(48):18574–18584. https://doi.org/10.1074/jbc.RA118.006052
Bernardo-García N, Mahasenan KV, Batuecas MT, Lee M, Hesek D, Petráčková D, Doubravová L, Branny P, Mobashery S, Hermoso JA (2018) Allostery, recognition of nascent peptidoglycan, and cross-linking of the cell wall by the essential penicillin-binding protein 2x of Streptococcus pneumoniae. ACS Chem Biol 13(3):694–702. https://doi.org/10.1021/acschembio.7b00817
Ytzhak S, Ehrenberg B (2014) The effect of photodynamic action on leakage of ions through liposomal membranes that contain oxidatively modified lipids. Photochem Photobiol 90(4):796–800. https://doi.org/10.1111/php.12266
Gurtovenko AA, Vattulainen I (2007) Ion leakage through transient water pores in protein-free lipid membranes driven by transmembrane ionic charge imbalance. Biophys J 92(6):1878–1890. https://doi.org/10.1529/biophysj.106.094797
Lee MT, Yang PY, Charron NE, Hsieh MH, Chang YY, Huang HW (2018) Comparison of the effects of daptomycin on bacterial and model membranes. Biochemistry 57(38):5629–5639. https://doi.org/10.1021/acs.biochem.8b00818
Wojda I, Alonso-Monge R, Bebelman JP, Mager WH, Siderius M (2003) Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways. Microbiology 149(5):1193–1204. https://doi.org/10.1099/mic.0.26110-0
Czövek P, Király I (2011) Inducible trehalase enzyme activity of Morchella conica Persoon mycelium. Acta Microbiol Immunol Hung 58(1):1–11. https://doi.org/10.1556/AMicr.58.2011.1.1
Cox SD, Mann CM, Markham JL (2001) Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol 91(3):492–497. https://doi.org/10.1046/j.1365-2672.2001.01406.x
Akram M (2014) Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68(3):475–478. https://doi.org/10.1007/s12013-013-9750-1
Tian J, Ban XQ, Zeng H, He JS, Chen YX, Wang YW (2012) The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One 7(1):e30147. https://doi.org/10.1371/journal.pone.0030147
Ma WB, Zhao LL, Zhao WH, Xie YL (2019) (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J Agric Food Chem 67(4):1138–1145. https://doi.org/10.1021/acs.jafc.8b06367
Wang CH, Wu SB, Wu YT, Wei YH (2013) Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med 238(5):450–460. https://doi.org/10.1177/1535370213493069
Saidana D, Boussaada O, Ayed F, Mahjoub MA, Mighri Z, Helal AN (2013) The in vitro free radical-scavenging and antifungal activities of the medicinal herb Limonium echioides L. growing wild in Tunisia. J Food Process Pres 37(5):533–540. https://doi.org/10.1111/j.1745-4549.2012.00673.x
Long DD, Fu RR, Han JR (2017) Tolerance and stress response of sclerotiogenic Aspergillus oryzae G15 to copper and lead. Folia Microbiol 62(4):295–304. https://doi.org/10.1007/s12223-017-0494-y
Cavalcanti Luna MA, Vieira ER, Okada K, Campos-Takaki GM, Nascimento AE (2015) Copper-induced adaptation, oxidative stress and its tolerance in Aspergillus niger UCP1261. Electron J Biotechnol 18(6):418–427. https://doi.org/10.1016/j.ejbt.2015.09.006
Lanubile A, Maschietto V, De Leonardis S, Battilani P, Paciolla C, Marocco A (2015) Defense responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol Plant Microbe In 28(5):546–557. https://doi.org/10.1094/MPMI-09-14-0269-R
Wang Y, Feng K, Yang H, Yuan Y, Yue T (2018) Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv 8(11):5806–5815. https://doi.org/10.1039/C7RA12191A
Sporer AJ, Kahl LJ, Price-Whelan A, Dietrich LEP (2017) Redox-based regulation of bacterial development and behavior. Annu Rev Biochem 86:777–797. https://doi.org/10.1146/annurev-biochem-061516-044453
Kumar KJS, Chu F-H, Hsieh H-W, Liao J-W, Li W-H, Lin JC-C, Shaw J-F, Wang S-Y (2011) Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol 136(1):168–177. https://doi.org/10.1016/j.jep.2011.04.030
Grintzalis K, Vernardis SI, Klapa MI, Georgiou CD (2014) Role of oxidative stress in sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Appl Environ Microbiol 80(18):5561–5571. https://doi.org/10.1128/AEM.01282-14
Zaccaria M, Ludovici M, Sanzani SM, Ippolito A, Cigliano RA, Sanseverino W, Scarpari M, Scala V, Fanelli C, Reverberi M (2015) Menadione-induced oxidative stress re-shapes the oxylipin profile of Aspergillus flavus and its lifestyle. Toxins 7(10):4315–4329. https://doi.org/10.3390/toxins7104315
Zhang F, Xu GP, Geng LP, Lu XY, Yang KL, Yuan J, Nie XY, Zhuang ZH, Wang SH (2016) The stress response regulator AflSkn7 influences morphological development, stress response, and pathogenicity in the fungus Aspergillus flavus. Toxins 8(7):202. https://doi.org/10.3390/toxins8070202
Marchetti P, Decaudin D, Macho A, Zamzami N, Hirsch T, Susin SA, Kroemer G (1997) Redox regulation of apoptosis: impact of thiol oxidation status on mitochondrial function. Eur J Immunol 27(1):289–296. https://doi.org/10.1002/eji.1830270142
Funding
This study was supported by the National Science Foundation of China (grand numbers 31871852, 31501575) and Natural Science Foundation of Henan Province (grand number 162300410047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, PP., Lv, YY., Lv, A. et al. Antifungal Effects of Fusion Puroindoline B on the Surface and Intracellular Environment of Aspergillus flavus. Probiotics & Antimicro. Prot. 13, 249–260 (2021). https://doi.org/10.1007/s12602-020-09667-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09667-2