Abstract
This study aims at evaluating the performance of thiamine as a new eco-friendly shale inhibitor in water-based drilling fluids (WBDFs). The evaluation experiments include sedimentation, bentonite inhibition, filtration, zeta potential, thermal gravimetric analysis, scanning electron microscopy, X-ray diffraction, shale cuttings recovery, linear swelling and Fourier transform infrared spectroscopy (FTIR). The performance of thiamine was compared to potassium chloride. In contrast to deionized water, the aqueous solution of thiamine exhibited greater power to inhibit montmorillonite (Mt) dispersion, much more Mt loading capacity (280 g/L) and fluid loss, lower Mt mass loss, larger aggregated Mt particles, lower interlayer space of the Mt particles, less shale cuttings disintegration and lower linear swelling. Adsorption of thiamine on Mt led to a significant shift in the value of zeta potential (from −17.1 to +8.54 mV). Thiamine demonstrated superior inhibitive performance than potassium chloride. FTIR analysis confirmed that thiamine is adsorbed on Mt particles. The compatibility test revealed the compatibility of thiamine with conventional WBDF additives. It was concluded that the main probable inhibition mechanisms of thiamine are the cation exchange and Mt surface coating. In view of its prominent inhibition capacity and great environmental acceptability, thiamine is a promising inhibitor for drilling in water-sensitive formations.
Similar content being viewed by others
1 Introduction
Drilling a well is the first stage and one of most costly stages of oil and gas production (Diaz-Perez et al. 2007). According to global statistics, the annual waste of money is estimated to be about $ 1 billion due to instability of oil and gas wells (Zeynali 2012). Design of a stable well is one of the important factors in the success of drilling operations (Cheatham Jr 1984; Fam and Dusseault 1998). Most researchers believe that the mechanisms of instability of wells are divided into two categories: mechanical effects and physicochemical effects (Tan et al. 1996; Osuji et al. 2008; Zeynali 2012). The mechanical effects are directly caused by drilling operations, which include the effects of drilling on the wall of the well (Zhu and Liu 2013). Mechanical effects can be controlled by controlling the well path and by restoring the balance of stress (Manohar Lal and Amoco 1999). Unlike mechanical effects, time-dependent physicochemical and chemical effects are caused by reaction between rock, especially shale, and drilling fluid (Zeynali 2012).
The clay minerals have two- and three-sheet structures. A clay mineral which consists of one tetrahedral sheet and one octahedral sheet is referred to as a 1:1 clay (the ratio of tetrahedral to octahedral sheet in a given unit layer), and the one which consists of a central octahedral sheet sandwiched between two tetrahedral sheets is called a 2:1 clay (Tamamura et al. 2014). Among all clay minerals, more attention is paid to sodium-saturated smectites (common weathering product) due to high swelling potential and also their abundance during drilling operations (Manohar Lal and Amoco 1999; Osuji et al. 2008; Zeynali 2012). Smectite is a three-sheet clay mineral in which an octahedral sheet is located between two tetrahedral sheets (Tamamura et al. 2014). Isomorphous substitution is a process that occurs during the formation of clay minerals through which the morphology of the clay minerals does not change, but the substitution gives the clay mineral a net negative charge that must be satisfied by exchangeable cations (Sun et al. 2015).
Montmorillonite (Mt), a member of the smectite groups in clay, is one of the favorite and useful minerals in the oil drilling industry. It has received more attention owing to several features such as altering the fluid rheology, cation exchange capacity (CEC) and swelling capacity (Agha et al. 2012). The phrase “sodium bentonite” in oil drilling industry is the public name, referred to as a mixture of minerals that are mainly composed of sodium-based Mt (Besq et al. 2003).
When water-sensitive shale is exposed to contact with conventional water-based drilling fluids, hydration and swelling of the shale occurs, resulting in many problems during drilling operations, such as stuck pipe, inappropriate cleaning of the well, bit balling, bit floundering, decrease in the quality of the logging data and cementing, creation of the fracture in the formation and blowout of the well (Bol et al. 1994; Manohar Lal and Amoco 1999; Lomba et al. 2000; Osuji et al. 2008). While oil-based drilling fluids have excellent performance against water-sensitive shaly formations, the use of oil-based drilling fluids in shaly formations due to the high price of these fluids and the destructive effects on the environment, especially in offshore drilling, is limited (Diaz-Perez et al. 2007). Therefore, design and production of water-based drilling fluids with a performance similar to the performance of oil-based drilling fluids for the application in water-sensitive shaly formations is one of the most important, interesting and today's research topics in the oil industry.
Many shale stabilizers have been introduced in the industry till now, which causes shale stability due to different mechanisms. A number of shale inhibitors are given in Table 1. The major disadvantages of shale stabilizers are high toxicity, high cost, corrosion and negative effects on rheological properties of drilling fluids. For example, water-based drilling fluids (WBDFs) containing high concentrations of K+ (> 1 wt%) fail the mysid-shrimp bioassay test. Therefore, fluids containing K+ are less acceptable in offshore drilling fluids (Anderson et al. 2010). High-molecular-weight quaternary amines are toxic, especially in offshore drilling fluids (Patel et al. 2007).
In the present study, for the first time a comprehensive study was performed on thiamine, as shale inhibitor, to evaluate its swelling inhibitive properties in the aqueous phase for water-based drilling fluids (WBDFs). The inhibitive effect of thiamine was also investigated using a wide range of inhibition characterization methods, including sodium bentonite inhibition, sodium bentonite sedimentation, filtration, zeta potential, thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), X-ray diffraction (XRD), cutting dispersion, dynamic linear swelling and compatibility tests. The Fourier transform infrared spectroscopy (FTIR) analysis was performed to confirm the adsorption of thiamine on Mt. In this study, we aimed to technically investigate the inhibition properties of thiamine through numerous tests performed on this inhibitor. It should be mentioned that the marketing of thiamine was not considered in this study. Except the compatibility test, the main purpose of the implemented tests was to examine the inhibition potential of thiamine. Therefore, the improvement in the drilling mud properties was not considered in the present study.
2 Methodology
2.1 Materials
2.1.1 Thiamine
Thiamine, also known as vitamin B1, is a water-soluble colorless organosulfur compound with the chemical formula of C12H17N4OS and molar mass of 265.35 g/mol, which can be found in food and produced as a dietary supplement and drug (Edwards et al. 2017). Its structure, shown in Fig. 1a, consists of an aminopyrimidine and a thiazole ring linked by a methylene bridge.
Thiamine structure: a in low pH, and b decomposed thiamine in alkaline pH (Edwards et al. 2017)
Thiamine is stable under acidic conditions and unstable in alkaline conditions. In the alkaline medium, the thiazole ring opens and turns into the thiol form (Fig. 1b). Potassium chloride is a common shale inhibitor, mostly applied in drilling shaly formations in the south of Iran. Therefore, in this study the inhibition potential of thiamine is only compared to that of potassium chloride. Both thiamine and potassium chloride were provided from the Kimia Pars Shayankar company.
2.1.2 Sodium bentonite
The mineral composition of the sample of sodium bentonite was identified using X-ray diffraction (XRD) analysis, which is shown in Table 2. The cation exchange capacity of the sample was 65.5 meq/100 g. In this study, sodium bentonite was employed as a representative of the swelling mineral of the shale in the wall of the well and not as a drilling mud additive. Sodium bentonite was provided from the Kimia Pars Shayankar company.
2.2 Experimental procedure
The experiments conducted to evaluate the inhibition potential of thiamine are presented in this section.
2.2.1 Sodium bentonite sedimentation tests
Sodium bentonite has a tendency to settle down in the inhibitive medium (medium in which there is a shale inhibitor). In the inhibitive medium, bentonite starts to settle down and exhibits low potential for hydration and swelling. Therefore, after a period of time, there is a distinct boundary between the clear zone and the depositions. Recording this boundary in terms of a function of time gives the appropriate index of bentonite potential for swelling in the inhibitive environment (Moslemizadeh et al. 2015). Sodium bentonite sedimentation test was investigated in both prehydrated and non-prehydrated conditions. In the prehydrated case, sodium bentonite was allowed to be hydrated in deionized water for 24 h. The sedimentation test consists of several steps as follows: (1) The solution of thiamine and sodium bentonite was prepared; in the prehydrated case, the thiamine powder was added to the prehydrated bentonite mixture; however, in the non-prehydrated case, the bentonite powder was added to the solution containing thiamine and deionized water. Following that, a certain amount of each of the prepared mixtures was poured into the test tubes. (2) The amount of sodium bentonite deposition was recorded by the h/H ratio in terms of a function of time, where h is the height of the sediments in cm and H is the total height of the mixture in cm. Deionized water was used in this paper to accurately investigate the thiamine inhibition properties without any effective factor.
In a real drilling operation, when water-sensitive shaly formation is exposed to WBDF, it becomes hydrated. This situation was simulated using non-prehydrated bentonite sample to investigate the inhibition potential of thiamine. The inhibition of sodium bentonite from being swollen in prehydrated condition is more difficult than that in non-prehydrated condition. Therefore, the sedimentation test was also conducted using the prehydrated sodium bentonite to indicate the high potential of thiamine to inhibit swelling and hydration of clay minerals.
2.2.2 Selecting optimum concentration
Determining the optimum concentration of thiamine as a shale inhibitor is economically important. Therefore, deposition test was used to determine the optimum concentration of thiamine. For this purpose, sodium bentonite sedimentation test was investigated in non-prehydrated condition. This test was performed at different concentrations of thiamine.
2.2.3 Sodium bentonite inhibition tests
The bentonite inhibition test is typically used as the chosen method to achieve the potential of a substance to prevent the swelling of bentonite and maintain a low rheological profile (Patel 2009). This method is designed to simulate the very active components of drilling solids in drilling fluid which allows one to gather information about what happens during drilling water-sensitive shaly formations. This test gives the maximum amount of bentonite that can be inhibited by shale inhibitors. The bentonite inhibition tests were carried out at two thiamine concentrations of 5 and 10 g/L in deionized water with adjusted pH of 9.5. To do so, a specified portion of thiamine was dissolved in 400 mL of deionized water to obtain the specified molar concentration. Then, 16 g of sodium bentonite was added to each solution, and the resulting mixture was stirred at an average shear rate for about 40 min to disperse bentonite in solutions completely. Each of the resulting suspensions was then poured into the aging vessel, and finally the vessel was hot rolled at 100 °C for 16 h. The temperature of 100 °C was set according to the temperature of the drilled shale formations. The samples were then cooled, and their dial readings were recorded at 60 °C using a viscosity meter (Fann Company, GA35 model). Rheological properties including apparent viscosity, plastic viscosity and yield point were measured. Apparent viscosity (AV), plastic viscosity (PV) and yield point (YP) were calculated from 300 and 600 rpm dial readings according to the API standard (API 1997). The aim of this test is to show the inhibition potential of thiamine by measuring the parameters AV, PV and YP. These parameters were not measured to calculate the rheological properties of the drilling fluids. Hence, there is no need for 10-s and 10-min gel strength in this test.
2.2.4 Filtration tests
Sodium bentonite has two main duties in water-based drilling fluids. It can be used as a viscosifier and as an agent for controlling fluid filtrates (Moslemizadeh et al. 2016). When sodium bentonite is exposed to an aqueous inhibitor solution, the potential of bentonite for hydration and swelling significantly reduces, which in turn leads to a high amount of filtration. According to this theory, recording the filtration of the API fluid can be an acceptable method to evaluate the potential of materials to inhibit clay from swelling. The procedure of this test is as follows: (1) 3.5 g of thiamine was added to 350 mL of deionized water, followed by addition of 7 g of sodium bentonite to this solution. The mixture was stirred at an average shear rate for 30 min to disperse sodium bentonite and then hot rolled for about 16 h at 100 °C (temperature of the drilled shale formations). (2) After 16 h, the amount of associated fluid filtrates was recorded by using an API fluid measuring device. To evaluate the inhibition potential of thiamine, this test was also performed by potassium chloride, as shale inhibitor, and without shale inhibitor, i.e., solution of sodium bentonite in deionized water (API 1997). These tests were conducted using the filter press Fann model 300.
2.2.5 Zeta potential measurements
The main driving force for adsorption of water to the clay mineral surface is the electrostatic force. Inhibitive materials can cover clay mineral surfaces and reduce the negative charge of clay mineral surface, which in turn prevent water from adsorbing into the clay. As a result, decrease in the negative charges on the surface of the clay mineral indicates the inhibition effect of the added material. The larger negative number of zeta potential indicates the higher degree of hydration and swelling of the clay mineral particles (Henry 1948). The procedure is as follows: Three types of samples were prepared; in the first sample, 2 g of sodium bentonite was dispersed in 100 mL deionized water, in the second sample, 0.5 g of thiamine was dissolved in 100 mL of deionized water followed by addition of 2 g sodium bentonite to the solution, and in the third sample, 1 g of thiamine was dissolved in 100 mL of deionized water followed by addition of 2 g sodium bentonite to the solution. The prepared samples were then placed individually in the stirrer for 24 h to disperse sodium bentonite completely. The conditions of the stirrer were the same for all the samples. After preparation of the samples, the zeta potential was measured for all the prepared samples by Malvern Zen 3600 Zetasizer at 25.1 °C.
2.2.6 Thermal gravimetric analysis (TGA)
One of the methods for investigating the swelling inhibition property of a substance is a thermal analysis method based on sample mass measurement during heating. This method provides useful information when the substance is decomposed during heating. The thermal weighing device is a sensitive electronic machine that measures the mass of the sample based on the variation of electrical current in a coil. TGA was used to check the effects of thiamine on the amount of water present in sodium bentonite. The TGA test was conducted to show that in the suspension containing thiamine, the adsorption of water on Mt is less than that in the thiamine-free suspension. The procedures of preparation of the samples were the same as those used in zeta potential analyses. In this test, after 24 h, each of the prepared samples was placed in a centrifuge at 5000 rpm for 2 h. The depositions were then placed in an oven at 105 °C for 24 h to be dried completely. The dried depositions of each sample were powdered and packaged individually, and the TGA analysis was performed on the prepared powder samples. This test was conducted using the simultaneous thermal analyzer Lenseis/L50/1750, at the scan rate of 15 °C/min under nitrogen flow.
2.2.7 Scanning electron microscopy (SEM) observations
SEM analysis is used to investigate the morphological feature of Mt particles when influenced by an inhibitor (Shadizadeh et al. 2015). For this reason, two types of samples of sodium bentonite in deionized water were prepared; one in the presence of thiamine, as the clay inhibitor, and the other without the presence of thiamine. The procedure of preparation of the samples was the same as the TGA analysis. In this case, similar to the TGA analysis, powdered and packed samples were also prepared. SEM analysis was then performed on each of the prepared powder samples using the VEGA\TESCAN-LMU model. This analysis was made at ambient temperature and atmospheric pressure.
2.2.8 Measurement of interlayer space with XRD
XRD is a precise and accurate technique for studying the properties of crystals. For this reason, XRD analysis was used in the present study to calculate the distance between a plan in a unit layer and the corresponding plan in the next unit layer in the structure of montmorillonite, denoted as d001 of layers in sodium bentonite (Zhong et al. 2013). To calculate this distance, the Bragg equation was used (Freiser and Freiser 1992):
where d001 is in (nm), \(\theta\) is the angle between the rays and the screen, λ represents the wavelength (nm) of the rays and n is an integer number. The powdered depositions from the samples containing sodium bentonite and deionized water in the presence of thiamine and without thiamine were prepared according to the sample preparation procedure for TGA. The analysis was performed using the XRD machine of the PHILIPS company, model PW1730 with the X-ray wavelength of λ = 1.54056 Å from a copper X-ray source.
2.2.9 Cuttings dispersion test
Cuttings dispersion test (resistant to shale crushing) is a common method for optimizing drilling fluid recommended by various laboratories and API institutes. This test simulates the behavior of cuttings being circulated up the wellbore (Friedheim et al. 2011). In order to perform this test, 20 g of shale cuttings between sieve mesh N.5 and N.10 dried at 105 °C were added to 350 mL of the prepared fluids. Three fluid samples were prepared for this experiment. The composition of the base fluid is equal to the composition of a real drilling fluid applied by National Iranian Oil Company (NIOC) to drill a well in a real field. The other two fluids were prepared by addition of potassium chloride and thiamine to the base fluid. Then, the prepared samples were hot rolled at 100 °C for 16 h (Fann, Model 704ET). The temperature of 100 °C was set according to the temperature of the drilled shale formations. The obtained fluids from hot rolling were screened with sieve mesh N.10 until the remaining cuttings were separated from the fluids. The separated cuttings were dried at 105 °C, and then, the percentage of the recovered cuttings was obtained for each fluid. The compositions of the prepared fluids are shown in Table 3. All the chemicals applied in this test were provided from the Kimia Pars Shayankar Company.
2.2.10 Dynamic linear swelling test
The dynamic linear swelling test is used to examine the interaction between reactive clays and WBDF while being circulated during drilling (Moslemizadeh et al. 2015). The dynamic linear swelling test consists of several steps as follows: (a) preparation of shale wafer using hydraulic compactor with 20 g of shale cuttings dried at 105 °C under the pressure of 41 MPa for 30 min; (b) placing the prepared shale wafer in linear swelling cup assembly (LSCA) located between the stirring hot plate and linear variable differential transducer; (c) pouring the prepared fluid to the LSCA; (d) putting thermocouple in the LSCA; (e) adjusting the stirring hot plate to the desired temperature and stirring the prepared sample; and (f) recording linear swelling data as a function of time. The compositions of the prepared fluids in this test are the same as those in the cutting dispersion test. In order to examine the performance of thiamine, this test was also performed using potassium chloride to make a comparison between the two inhibitors. The tests were conducted at 28 °C and atmospheric pressure condition. The compositions of the prepared fluids are shown in Table 3.
2.2.11 Fourier transform infrared spectroscopy (FTIR) analysis
Fourier transform infrared spectrometry is a measurement technique in which spectra are obtained from compounds based on measurements of radiation source coherence. This analysis was performed to confirm the adsorption of thiamine, as the clay inhibitor, on sodium bentonite (Zhong et al. 2013). The sample preparation method for this test was the same as TGA. The FTIR test was performed using an ATR-IR spectrometer (Bruker/model Tensor 27) with the spectrum in the range of 400–4000 cm−1 and the resolution of 1–4 cm−1.
2.2.12 Compatibility test
The compatibility of a shale inhibitor with other conventional additives that may be present in WBDFs is a vital characteristic of the inhibitor. In order to investigate the compatibility of thiamine with conventional WBDF additives, the compatibility tests were performed using samples No. 1 and No. 3 of Table 3 and samples No. 4 and No. 5 of Table 4. Samples No. 3 and No. 5 were prepared by addition of 10 wt% of thiamine to the prepared WBDF samples, denoted as samples No. 1 and No. 4, respectively. The formulations of the two WBDF samples used in this test are shown in Table 4. Similar to the cutting dispersion test, the compositions of the fluids used in this test are equal to the compositions of the drilling fluids applied by NIOC to drill a well in a real field. The polymeric water-based muds were used as the drilling fluids in this field. The fluid samples were aged for 4 h at specified temperatures in rolling oven, and finally their rheological properties and filter loss were measured before and after addition of thiamine. All the chemicals used in this test were provided from the Kimia Pars Shayankar Company.
2.2.13 Reflux test
The reflux test was used to evaluate the inhibition potential of thiamine in alkaline and high temperature solutions. A Soxhlet extractor was employed to perform the reflux test. The reflux extraction test was carried out using the aqueous solution of thiamine in deionized water with the concentration of 10 g/L and the adjusted pH of 9.5. To do so, the specified portion of thiamine was dissolved in 250 mL of deionized water with the pH of 9.5 to obtain the desired concentration. The resulting mixture was then stirred at an average shear rate of 200 rpm to completely dissolve thiamine in the solution. Following that, the resulting solution was poured into a balloon. The balloon was heated until the solution began to boil. The vapor liberated from this solution was cooled by a condenser and collected in the extractor thimble. The condensate collected in the extractor thimble was returned to the balloon and vaporized again. This process was continued for 8 h. Finally, the solution collected in the balloon was cooled to the room temperature.
3 Results and discussion
The results obtained from different analyses to investigate the inhibition performance of the proposed material are presented in this section.
3.1 Sedimentation test results
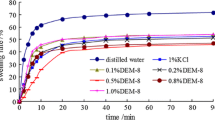
The deposition behavior of sodium bentonite in the presence of thiamine is shown in Figs. 2 and 3 for the non-prehydrated and prehydrated sodium bentonite particles, respectively. The experiments were also performed using deionized water without the presence of thiamine as the inhibitor. When sodium bentonite particles are added to deionized water, a stable dispersed suspension of bentonite particles is created. The created uniformly dispersed suspension in the graduated cylinder is cloudy (Figs. 2, 3). By addition of sodium bentonite particles to the prepared solutions of thiamine in deionized water with the thiamine concentrations of 1, 5 and 10 g/L, thiamine particles are adsorbed to the sodium bentonite particles. By adsorption of thiamine to the non-prehydrated sodium bentonite particles, they are prevented from being dispersed in the solution and the particles immediately start to precipitate (Fig. 2b). As the concentration of thiamine in the solution increases, the tendency of sodium bentonite particles to settle down would also increase. This phenomenon can be observed by disappearing the cloudy region in the prepared aqueous solutions of non-prehydrated sodium bentonite in the presence of thiamine. Therefore, disappearance of the cloudy solution can be a good indication of the sediments formed at the bottom of the graduated cylinder. The rate at which sedimentation occurs can be measured from the variations in length of the cloudy region, h, to the total length of the solution, H, in the graduated cylinder. The ratios of h/H versus time are depicted in Figs. 2 and 3 for both non-prehydrated and prehydrated sodium bentonite aqueous mixtures in the presence of thiamine. The base case, denoted by deionized water, is a uniformly dispersed suspension of sodium bentonite particles in which the h/H remains constant at 1.0 over the time period investigated. By addition of sodium bentonite to the aqueous solutions containing thiamine, the h/H ratio decreases rapidly and reaches a constant value after about 24 h (Fig. 2a). The stable value of h/H depends on the thiamine concentration in the solution, which is decreased by increasing the concentration of thiamine. The rapidly varying region in Fig. 2a is enlarged for clarity (Fig. 2b). When thiamine with different concentrations is added to the aqueous mixtures of sodium bentonite, i.e., the prehydrated cases, the same phenomenon occurs. However, in this case, the rate of decrease in h/H ratio is slower than the non-prehydrated cases. At initial times of the experiments, no significant change is observed in the h/H ratio. In addition, the stabilized value of h/H ratio is about 1.4–2.9 times higher than the corresponding value of the non-prehydrated cases. In cases where sodium bentonite particles are prehydrated, the deposition behavior of the solution containing 1 g/L thiamine is comparable with that containing 5 g/L thiamine (Fig. 3). According to the results, the samples containing non-prehydrated sodium bentonite are less stable than those containing prehydrated particles of sodium bentonite. This is due to the larger sizes of the non-prehydrated sodium bentonite particles created in the presence of thiamine (Wang et al. 2011). Therefore, the inhibition potential of thiamine is much higher in cases where non-prehydrated sodium bentonite particles are present in the sample.
3.2 Optimum concentration of thiamine
The deposition behavior of sodium bentonite in the presence of different concentrations of thiamine is shown in Fig. 4. The results showed that the sedimentation rate increases by increasing the concentration of thiamine. However, in the concentration range between 10 g/L and 15 g/L, the increase in thiamine concentration has no significant effect on the amount and rate of sedimentation and the results are so close together. Hence, 10 g/L is selected as the optimum and economical concentration of thiamine as the shale inhibitor.
3.3 Inhibition test results
Inhibition test is one of the most interesting methods to reveal the inhibition properties of a particular additive by analyzing the rheological properties versus the amount of sodium bentonite content. The lower rheological profile of sodium bentonite in a particular fluid indicates that sodium bentonite is less swollen and is stable (Hafshejani et al. 2016).
Plastic viscosity represents the viscosity resulting from the high shear rate and is a function of the liquid phase viscosity and the volume of solids in drilling muds. Plastic viscosity increases with the addition of any solids to the mud (Moslemizadeh et al. 2019). However, solids such as clay that swell due to water adsorption can significantly increase the plastic viscosity.
On the other hand, yield point is a good indication of an additive potential to change the low shear rate viscosity. Yield point demonstrates the tendency of the clay mineral plates to cluster together and form a clay mineral structure. It means that if more plates are connected together, the yield point would be greater. In cases where Mt becomes swollen and layered, the number of plates in the fluid increases, which in turn increases the plastic viscosity. Due to the fact that the faces of the layered particles have negative charges with positive charges on the edges, the particles become more strongly connected together (face to edge connection). Consequently, the yield point of the solution increases as well (Annis and Smith 1996).
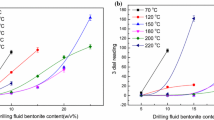
The effect of the concentration of thiamine, as an inhibitive additive, on the rheological properties of sodium bentonite suspension was studied using inhibition test. Different concentrations of thiamine were used to evaluate its inhibition properties. Inhibition potential of thiamine was also compared with that of potassium chloride at the same concentrations of sodium bentonite. The apparent viscosity, plastic viscosity and yield point, measured at different concentrations of sodium bentonite, are shown in Fig. 5a–c, respectively.
The sample obtained from the dispersion of 16 g sodium bentonite in deionized water without the inhibition additive was viscose and sticky, which exhibited higher rheological profile than the other samples in the presence of thiamine and potassium chloride (Fig. 5).
In the dry sodium bentonite, the Mt particles are arranged in stack form. When these particles are exposed to deionized water, they quickly adsorb water into the layers (Annis and Smith 1996; Moslemizadeh et al. 2016). This process causes an increase in d001 value. In this expanded state, blending, i.e., creating shear rate, causes more particles delamination. Therefore, more increase in the viscosity at both high and low shear rate values is observed (Fig. 5b, c).
In the presence of inhibitor, represented by the other three systems in Fig. 5, the rheology profiles change at the lower rate in comparison with the system containing only sodium bentonite and deionized water. According to the results, by variation in the concentration of sodium bentonite, the changes in the rheological properties of the sample containing 5 g/L thiamine are higher than that containing 10 g/L thiamine. In this regard, the samples containing 5 and 10 g/L thiamine undergo severe changes in rheological properties in the concentration range of sodium bentonite above 160 and 280 g/L, respectively (Fig. 5). It can be concluded that the inhibition potential of thiamine is influenced by its concentration and increases with increasing the inhibitor concentration.
For potassium chloride sample, the rate of changes in the rheological properties versus sodium bentonite concentration increases significantly in the concentration range above 240 g/L. Although this variation in the rheological properties occurs at the higher values of sodium bentonite concentration in comparison with the sample containing 5 g/L thiamine, in this case, the sample has much higher amount of the potassium chloride inhibitor, i.e., six times higher.
The superior inhibition potential of thiamine is revealed when comparing the variations of rheological properties of the sample containing 10 g/L thiamine with those of the sample containing 30 g/L potassium chloride, as illustrated in Fig. 5. The concentration of thiamine in the former sample is one-third of the inhibitor concentration, i.e., potassium chloride in the later sample. However, the rheological properties, i.e., apparent viscosity, plastic viscosity and yield point of the thiamine-containing sample, vary significantly at the higher values of sodium bentonite concentration than those of the sample containing potassium chloride inhibitor. According to the inhibition test results, thiamine exhibits superior performance than potassium chloride as an inhibitor and can be considered as a highly efficient clay stabilizer.
In the solution containing thiamine, when sodium bentonite is added to the solution, thiamine is adsorbed to the sodium bentonite particles and prevents them from swelling (Fig. 5). This phenomenon limits the amount of water that sodium bentonite particles can adsorb and makes them stable through the possible mechanism of cation exchange. The cation exchange causes an electrostatic gravity. The electrostatic gravity prevents the separation of the layers of sodium bentonite and hence makes it stabilized. When Mt particles become stable, the total volume and number of the layers in thiamine sample are reduced. As a result, plastic viscosity decreases. By decreasing the layers and neutralizing positive charges in the separated layers, these layers cannot be connected to each other. Subsequently, yield point also decreases in the presence of thiamine.
3.4 Filtration measurement results
The ability of sodium bentonite particles to be hydrated in fresh water is one of the important factors in controlling the amounts of filtrates. To do so, sodium bentonite particles were exposed to deionized water and aqueous solutions of thiamine and potassium chloride. Then, these samples were hot rolled at 100 °C for 16 h and filtrated. According to the measured results of filtrates, the sodium bentonite sample in deionized water with the filtrate amount of 32 mL in 30 min has the lowest amount of filtrate among the prepared samples (Fig. 6). This is due to the fact that sodium bentonite can easily be hydrated and swollen in pure water. When thiamine or potassium chloride is present in the aqueous solution, the added bentonite particles are prevented from swelling and being dispersed in the suspension. Therefore, the amounts of filtrates in the later cases increase significantly. In addition, the amounts of filtrates in the presence of thiamine with the concentration of 10 g/L are higher than that of potassium chloride with the concentration of 30 g/L, which again confirms the higher inhibition potential of thiamine compared with potassium chloride (Fig. 6).
3.5 Zeta potential results
Zeta potential, which indicates the charges on the plates of clay mineral, was used to measure the stability of sodium bentonite particles in the aqueous solution of thiamine. Sodium bentonite suspension in deionized water has a high negative zeta potential of −17.1 mV (Table 5). By addition of sodium bentonite to the aqueous solutions of thiamine with the concentrations of 5 and 10 g/L, the zeta potential varies from negative value to the positive values of +3.61 and +8.54 mV, respectively (Table 5). The positive zeta potential of the aqueous suspension of sodium bentonite in the presence of thiamine arises from the intercalation of cations of thiamine into the interlayer space of Mt (Moslemizadeh et al. 2016). Therefore, the repulsive forces between the plates decrease and the particles are prevented from swelling and delaminating.
3.6 TGA results
During the process of swelling, i.e., crystalline and osmotic swelling, the voluminous amount of water is adsorbed into the Mt structure. It has been reported that mass loss of clay minerals because of evaporation of physically adsorbed water and dehydration of interchangeable hydrated cations, such as sodium ion, can occur at temperatures lower than 200 °C (Moslemizadeh et al. 2016). The mass loss at temperatures between 200 and 500 °C is due to the decomposition of organic compounds (Barick and Tripathy 2010). The mass loss of Mt in the temperature range from ambient to 200 °C shows the amount of water adsorbed to the particles. The mass loss of the Mt powder obtained from its suspension in pure deionized water is 3.07%, which is 3.9 times higher than that obtained from the aqueous solution of thiamine, i.e., 0.79% mass loss (Fig. 7). Therefore, in the presence of thiamine, the amount of water adsorbed to the sodium bentonite particles is reduced significantly. In the temperature range above 200 °C, the sodium bentonite powder obtained from the aqueous solution of thiamine has the larger mass loss compared to that obtained from deionized water, which arises from the fact that in this temperature range the adsorbed thiamine on the sodium bentonite particles is decomposed (Fig. 7).
3.7 SEM morphology
The morphology behavior of sodium bentonite particles highly depends on the inhibition potential of its aqueous suspension. According to the SEM observations, when sodium bentonite particles are in contact with pure deionized water, the size of the particles becomes smaller and a tighter texture is formed compared with the morphology of the particles created in the presence of the aqueous solution of thiamine (Fig. 8). SEM images are shown with two different magnifications for each sample. As it was expected, the difference observed in the morphology of sodium bentonite particles in the two samples arises from the fact that in the pure deionized water, there is a higher tendency of the particles to be hydrated and swollen (Fig. 8a1, a2). In contrary, in the presence of the aqueous solution of thiamine with the inhibitor concentration of 10 g/L, due to the adsorption of thiamine to sodium bentonite particles, swelling and delamination of the particles decrease significantly, which lead to the creation of the coarser particles (Fig. 8b1, b2).
3.8 Interlayer space of sodium bentonite sample
The amount of swelling of clay can be controlled by inhibitive solutions, which make the layers of the clay mineral remain closer together. Swelling is calculated directly by measuring the d001 value. According to the results of the XRD analysis (Fig. 9) and by using the Bragg relationship, the interlayer space of sodium bentonite in deionized water is calculated as 1.30 nm, while this value is obtained as 1.23 nm in the aqueous solution of thiamine, close to that calculated for the dry sample of sodium bentonite without any treatment (1.2 nm). Therefore, thiamine can reduce the space between the layers very well and hence decrease the amount of sodium bentonite swelling.
3.9 Cuttings recovery
The inhibition potential of thiamine and potassium chloride was determined by cuttings dispersion test with highly reactive shale cuttings (Fig. 10). The recovery rate in the drilling fluid without any inhibitor (fluid No.1 of Table 3) was 62.37%, representing high hydration and swelling potential of shale cuttings. After addition of 10.5 g potassium chloride and 3.5 g thiamine to the drilling fluid, the recovery rate increased to 77.62% and 88.14%, respectively. This indicates that thiamine has a considerable ability to inhibit hydration and swelling of shale cuttings. Moreover, as an inhibitor, thiamine exhibits superior performance than potassium chloride and can be considered as a highly efficient shale stabilizer. Therefore, the results showed that thiamine can act as an excellent shale inhibitor in WBDFs.
3.10 Linear swelling test results
The linear swelling test was performed using the prepared fluids with the compositions as shown in Table 3. The results are presented in Fig. 11. As the time proceeds, the slope of the curves decreases until the linear swelling reaches a constant equilibrium value, except for the inhibitor-free fluid (fluid No. 1) curve (Fig. 11). According to Fig. 11, the constant equilibrium value at which the swelling rate approaches zero is not observed for the inhibitor-free fluid. The swelling amount in the inhibitor-free fluid is larger than that in the other two fluids. The results also indicate that the swelling amount in the fluid containing thiamine (fluid No. 3) is less than that in the other two fluids. The constant equilibrium values of swelling in the fluids containing thiamine and potassium chloride are obtained after 16 and 23.5 h, respectively. The results of the dynamic linear swelling test again confirm the excellent inhibition potential of thiamine in comparison with potassium chloride.
3.11 FTIR analysis
The FTIR analysis was used to obtain infrared spectra of sodium bentonite, thiamine and thiamine-modified sodium bentonite. The major absorption peaks of sodium bentonite include stretching of structural hydroxyl groups (3626.83 cm−1), broad stretching band of water (3443.73 cm−1), Si–O stretching band (1087.62 cm−1 and 1036.46 cm−1), deformation band of water (1638.3 cm−1), coupled out-of-plan vibration band of Al–O and Si–O (624.71 cm−1), Si–O stretching band of silica (794.13 cm−1), deformation band of Al–O–Si (520.49 cm−1) and deformation band of Si–O–Si (467.88 cm−1) (Fig. 12a) (Ritz et al. 2011).
The absorption peaks of thiamine occur at 3451.86 cm−1 attributed to OH bending, 1639.07 cm−1 attributed to NH2 bending vibration and 1383.57 cm−1 attributed to N cation (Fig. 12b). However, in the spectra of thiamine-modified sodium bentonite, new peaks are detected, which indicates the adsorption of thiamine on sodium bentonite and to some extent the interaction between them (Fig. 12c). The new absorption peaks of the thiamine-modified sodium bentonite include C–H bonds stretching vibration (2923.97 cm−1 and 2854.6 cm−1), C=O stretching vibration (1736.11 cm−1), N–H deformation vibration (1566.7 cm−1), C–C stretching band (1448.94 cm−1) and C–H stretching vibration (1373.37 cm−1). The shift in the absorption peak of thiamine in the thiamine-modified sodium bentonite reveals the presence of substantial hydrogen bonds and cation exchange, which results from the intercalation of thiamine to the interlayer space of sodium bentonite particles (Greenwell et al. 2005). The created hydrogen bonds and cation exchange demonstrate that the molecules of thiamine were adsorbed on clay particles.
3.12 Compatibility test results
The compatibility tests were performed to achieve compatibility between thiamine and conventional WBDF additives. The use of special materials in WBDFs is strongly dependent on their compatibility with other additives. The compatibility is revealed by measuring the rheological properties and filtration value of the fluid samples before and after addition of thiamine. Undoubtedly, high deviations between the measured data of modified (with thiamine) and non-modified (without thiamine) fluids indicate that thiamine is incompatible with other conventional WBDFs additives. The results of compatibility tests (ratio of each property of modified fluid to the non-modified one) are shown in Table 6. All the presented data are close to unity, indicating that thiamine does not have a significant effect on the rheological properties of the designed fluids. Another important result of this test is that thiamine has a positive impact on fluid loss reduction because the calculated data for fluid loss ratio are less than unity for all the samples. Reduction in fluid loss of the drilling fluid is favorable and can be an index of improving the drilling fluid properties. The results of this test show that due to no significant deviation in the measured data, thiamine is compatible with other conventional additives of WBDF and can improve drilling fluid properties such as the amount of fluid loss.
3.13 Evaluation of refluxed samples
In order to evaluate the inhibition properties of thiamine in alkaline and high temperature solution, sodium bentonite sedimentation test was performed using the solution obtained from the reflux test rather than the aqueous solution of thiamine. The procedure of preparation of the solution samples was the same as those used in sodium bentonite sedimentation test. The results revealed the sedimentation of sodium bentonite in this case (Fig. 13). This experimental result indicates that thiamine can still prevent swelling of the shale in high temperature and alkaline solution. The reason for the inhibition characteristic of thiamine in this condition is the OH and NH2 molecules existing in the structure of thiamine (Edwards et al. 2017).
FTIR analysis was also performed for better investigation of the thiamine behavior in neutral solution with ambient temperature and alkaline solution at high temperature. The results indicated that the absorption peaks of thiamine occur at 3451.86, 1639.07 and 1383.57 cm−1 attributed to OH bending, NH2 bending vibration and N cation, respectively (Fig. 14). The absorption peaks of solution occur at 3448.01 and 1637.67 cm−1 attributed to OH bending and NH2 bending vibration, respectively (Fig. 15).
4 Inhibition mechanisms of thiamine
Thiamine is a colorless organosulfur compound with the chemical formula of C12H17N4OS. Its structure consists of an aminopyrimidine and a thiazole ring linked by a methylene bridge. The results of the experiments indicated that thiamine has an inhibition property and prevents clay from hydration. Thiamine is stable under acidic conditions, but it is unstable in alkaline condition (Pattison et al. 2007). Also, the increase in temperature increases the rate of the instability of thiamine. The inhibition mechanism of thiamine depends on the pH of the solution, which is investigated in this section.
4.1 Neutral and acidic conditions
As it was mentioned above, thiamine is stable under acidic conditions. To better understand the underlying mechanism, the behavior of Mt in deionized water and aqueous solution of thiamine is indicated graphically by Parvizi Ghaleh et al. (2020). Thiamine has an N+ cation in its structure. When it is dissolved in water, NH3+ cation is created as well (Reedy 1929). When Mt is dispersed in aqueous solution of thiamine, the thiamine cations rather than water molecules are adsorbed to the interlayer space of Mt through the various driving forces such as ion exchange, electrostatic gravity and large amount of positive charges (Xuan et al. 2013). These positive charges are replaced by charge balancing cations. The other inhibition mechanism is the formation of hydrogen bonding between OH group and the surface of Mt particles. Then, the clay mineral planes become firmly attached to each other due to the hydrogen bonding and their charge equilibrium. As a result, thiamine prevents the clay from swelling and dispersion. The proposed mechanism can be considered as a valid reason for the inhibition effect of thiamine. The important point in here is that actually most of the drilling fluids are alkaline. Hence, the above-mentioned mechanism is not applicable for real drilling operations.
4.2 Alkaline conditions
In an alkaline solution, the thiamine structure is opened and the thiazole ring converts to the thiol form (Fig. 1b). Also, an increase in the temperature increases the rate of instability of thiamine (Edwards et al. 2017). In this condition (alkaline condition with high temperature), the thiamine structure changes; however, NH2 and OH exist in the obtained structure (Fig. 1b). Therefore, as mentioned previously, the clay mineral is stabilized through the two mechanisms, cation exchange and hydrogen bonding creation (Parvizi Ghaleh et al. 2020). In other words, the inhibition behavior of thiamine is maintained, despite its structure is broken. In alkaline medium, the stability of the clay mineral in the presence of thiamine was also confirmed through several experiments, including sodium bentonite inhibition tests, cuttings dispersion test, dynamic linear swelling test and reflux test. It should be mentioned that in drilling shaly formations, the drilling fluids are applied in alkaline and high temperature conditions. Therefore, the mechanisms described in this section are applicable for real drilling operations.
5 Cost and environmental assessment of thiamine
Cost and toxicity are two main factors to the applicability of shale inhibitors. Thiamine is a popular vitamin, and its application is primarily limited to food and medicine industries. Also, the material safety data sheet of thiamine and several studies show that this vitamin is biodegradable and nontoxic for human and environment (Ghafuri et al. 2016; Institute OEnologique de Champagne 2010; Kusampally et al. 2019). In a large-scale request, the industry grade of thiamine is economically feasible with an average price of $ 8–12 per kilogram, which can be broadly found by many companies worldwide. Therefore, it can be claimed that there are no limitations on the availability of this product.
In addition to the acceptable technical performance, thiamine also has the prominent environmental friendliness property. This property allows making use of thiamine as a safe shale inhibitor in water-based drilling fluids. Some additives have been reported for use in WBDFs that are not environmentally compatible and cause many environmental problems (Patel et al. 2007). In the present study, thiamine was proposed as a new shale inhibitor which can be found in food and produced as a dietary supplement and drug. Some of the features of thiamine are as follows: Thiamine as a vitamin B1 is found in many foods including yeast, cereal grains, beans, nuts and meat. It has many medical applications and is used for treatment of many diseases, including preventing cancer and progression of kidney disease in patients with type 2 diabetes (Fattal-Valevski 2011). Thiamine is also used for digestive problems including poor appetite and ulcerative colitis. It is essential for the health of human beings and animals and is present in all organisms. Biological studies showed that thiamine is a vital vitamin for the growth of lots of microorganisms (Edwards et al. 2017). Due to its applications and useful properties, thiamine has no harmful effects on the environment and hence, can be used in drilling muds.
6 Conclusions
In this study, for the first time, thiamine was assessed as an eco-friendly shale inhibitor in WBDFs. This goal was achieved by carrying out a number of experiments. Below are the conclusions that can be drawn from this study:
-
1.
The results from inhibition evaluation experiments implied that thiamine is strongly capable of hindering clay swelling. Thiamine was found to be more inhibitive than potassium chloride.
-
2.
Thiamine promotes sodium bentonite to flocculate in water.
-
3.
The compatibility of thiamine with conventional WBDF additives was observed from the results of the compatibility test.
-
4.
FTIR analysis confirmed the adsorption of thiamine on sodium bentonite.
-
5.
It was concluded that the main probable inhibition mechanisms of thiamine are the cation exchange (thiamine cations intercalate the interlayer space of Mt) and Mt surface coating by the formation of hydrogen bonding between OH group and the surface of Mt particles.
-
6.
The results from different tests on the proposed inhibitor allow one to conclude that thiamine has the prominent potential to prevent swelling and hydration of clay, and it can be used as a strong and environmentally friendly clay inhibitor.
References
Agha M, Ferrell RE, Hart GF. Mineralogy of Egyptian bentonitic clays I: discriminant function analysis. Clay Miner. 2012;60(4):387–404. https://doi.org/10.1346/CCMN.2012.0600405.
Akhtarmanesh S, Shahrabi M, Atashnezhad A. Improvement of wellbore stability in shale using nanoparticles. J Pet Sci Eng. 2013;112:290–5. https://doi.org/10.1016/j.petrol.2013.11.017.
An Y, Jiang G, Ren Y, Zhang L, Qi Y, Ge Q. An environmental friendly and biodegradable shale inhibitor based on chitosan quaternary ammonium salt. J Pet Sci Eng. 2015;135:253–60. https://doi.org/10.1016/j.petrol.2015.09.005.
Anderson RL, Ratcliffe I, Greenwell HC, Williams PA, Cliffe S, Coveney PV. Clay swelling—a challenge in the oilfield. Earth-Sci Rev. 2010;98(3–4):201–16. https://doi.org/10.1016/j.earscirev.2009.11.003.
Annis MR, Smith V. Drilling fluids technology. Revised edition. Irving: Exxon Company; 1996.
API, RP 13B-1 recommended practice standard procedure for field testing water based drilling fluids. Americal Petroleum Institute Publishing Services, USA. September 1, 1997.
Barati P, Keshtkar S, Aghajafari A, Shahbazi K, Momeni A. Inhibition performance and mechanism of Horsetail extract as shale stabilizer. Pet Explor Dev. 2016;43(3):522–7. https://doi.org/10.1016/S1876-3804(16)30061-1.
Barick A, Tripathy D. Thermal and dynamic mechanical characterization of thermoplastic polyurethane/organoclay nancomposites prepared by melt compounding. Mater Sci Eng. 2010;527(3):812–23. https://doi.org/10.1016/j.msea.2009.10.063.
Besq A, Malfoy C, Pantet A, Monnet P, Righi D. Physicochemical characterization and flow properties of some bentonite muds. Appl Clay Sci. 2003;23(5–6):275–86. https://doi.org/10.1016/S0169-1317(03)00127-3.
Bol G, Wong SW, Davidson C, Woodland D. Borehole stability in shales. SPE Drill Complet. 1994;9(2):87–94. https://doi.org/10.2118/24975-PA.
Cheatham JB Jr. Wellbore stability. J Pet Technol. 1984;36(6):889–96. https://doi.org/10.2118/13340-PA.
Chegny SJ, Tahmasbi K, Arsanjani N. The possibility of replacing OBMs with emulsified glycol mud systems in drilling low-pressure zones of Iranian oilfields. Presented at the IADC/SPE Asia Pacific drilling technology conference, 25–27 August, Jakarta, Indonesia. 2008. https://doi.org/10.2118/114067-MS.
Chen G, Yan J, Li L, Zhang J, Gu X, Song H. Preparation and performance of amine-tartaric salt as potential clay swelling inhibitor. Appl Clay Sci. 2017;138:12–6. https://doi.org/10.1016/j.clay.2016.12.039.
Diaz-Perez A, Cortes-Monroy I, Roegiers J. The role of water/clay interaction in the shale characterization. J Pet Sci Eng. 2007;58(1–2):83–98. https://doi.org/10.1016/j.petrol.2006.11.011.
Edwards KA, Tu-Maung N, Cheng K, Wang B, Baeumner AJ, Kraft CE. Thiamine assays—advances, challenges, and caveats. Chem Open. 2017;6(2):178–91. https://doi.org/10.1002/open.201600160.
Fam M, Dusseault M. Borehole stability in shales: a physico-chemical perspective. In: SPE/ISRM rock mechanics in petroleum engineering. 8–10 July, Trondheim, Norway. 1998. https://doi.org/10.2118/47301-MS.
Fattal-Valevski A. Thiamine (vitamin B1). J Evid Based Complement Altern Med. 2011;16(1):12–20. https://doi.org/10.1177/1533210110392941.
Freiser H, Freiser M. Concepts calculations in analytical chemistry, featuring the use of Excel. Roca Raton: CRC Press; 1992.
Friedheim J, Guo Q, Young S, Gomez S. Testing and evaluation techniques for drilling fluids–shale interaction and shale stability. In: The 45th US rock mechanics/geomechanics symposium, 26–29 June, San Francisco, California, USA. 2011. ARMA 11-502.
Ghafuri H, Joorabchi N, Keshvari Kenari M, Emami A. Thiamine: an efficient, biodegradable, green catalyst for one-pot synthesis of functionalized dihydropyridines. In: The 20th international electronic conference on synthetic organic chemistry, November 2016. https://doi.org/10.3390/ecsoc-20-a039.
Greenwell HC, Harvey MJ, Boulet P, Bowden AA, Coveney PV, Whiting A. Interlayer structure and bonding in nonswelling primary amine intercalated clays. Macromolecules. 2005;38(14):6189–200. https://doi.org/10.1021/ma0503817.
Hafshejani KS, Moslemizadeh A, Shahbazi K. A novel bio-based deflocculant for bentonite drilling mud. Appl Clay Sci. 2016;127:23–34. https://doi.org/10.1016/j.clay.2016.03.037.
Henry D. The electrophoresis of suspended particles. IV. The surface conductivity effect. Trans Faraday Soc. 1948;44:1021–6. https://doi.org/10.1039/TF9484401021.
Institut OEnologique de Champagne. Safety data sheet. 2010.
Kusampally U, Soma R, Kamatala CR. Thiamine hydrochloride as efficient catalyst for one-pot synthesis of 14-aryl-14 h dibenzo [a, j] xanthenes under greenery conditions. Rasayan J Chem. 2019;12(1):152–6. https://doi.org/10.31788/RJC.2019.1215043.
Ji L, Guo Q, Friedheim JE, Zhang R, Chenevert ME, Sharma MM. Laboratory evaluation and analysis of physical shale inhibition of an innovative water-based drilling fluid with nanoparticles for drilling unconventional shales. In: SPE Asia Pacific oil and gas conference and exhibition, 22–24 October, Perth, Australia. 2012. https://doi.org/10.2118/158895-MS.
Li G, Zhang J, Hou Y. Nanotechnology to improve sealing ability of drilling fluids for shale with micro-cracks during drilling. In: SPE international Oilfield nanotechnology, 12–14 June, Noordwijk, Netherland. 2012. https://doi.org/10.2118/156997-MS.
Lomba RF, Chenevert M, Sharma MM. The ion-selective membrane behavior of native shales. J Pet Sci Eng. 2000;25(1–2):9–23. https://doi.org/10.1016/S0920-4105(99)00028-5.
Luo Z, Wang L, Yu P, Chen Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl Clay Sci. 2017;150:267–74. https://doi.org/10.1016/j.clay.2017.09.038.
Manohar Lal S, Amoco B. Shale stability: drilling fluid interaction and shale strength. In: Asia Pacific oil and gas conference, 21–23 April, Caracas, Venezuela. 1999. https://doi.org/10.2118/54356-MS.
Moslemizadeh A, Shadizadeh SR. Minimizing water invasion into kazhdumi shale using nanoparticles. Iran J Oil Gas Sci Technol. 2015;4(4):15–32. https://doi.org/10.22050/ijogst.2016.12475.
Moslemizadeh A, Shadizadeh SR, Moomenie M. Experimental investigation of the effect of henna extract on the swelling of sodium bentonite in aqueous solution. Appl Clay Sci. 2015;105:78–88. https://doi.org/10.1016/j.clay.2014.12.025.
Moslemizadeh A, Aghdam SKY, Shahbazi K, Aghdam HKY, Alboghobeish F. Assessment of swelling inhibitive effect of CTAB adsorption on montmorillonite in aqueous phase. Appl Clay Sci. 2016;127:111–22. https://doi.org/10.1016/j.clay.2016.04.014.
Moslemizadeh A, Aghdam SKY, Shahbazi K, Zendehboudi S. A triterpenoid saponin as an environmental friendly and biodegradable clay swelling inhibitor. J Mol Liq. 2017;247:269–80. https://doi.org/10.1016/j.molliq.2017.10.003.
Moslemizadeh A, Samadzadeh Hafshejani K, Shahbazi K, Zaravi Dezfuli M, Zendehboudi S. A biosurfactant for inhibiting clay hydration in aqueous solutions: applications to petroleum industry. Can J Chem Eng. 2019;97:384–94. https://doi.org/10.1002/cjce.23172.
O’Brien DE, Chenevert ME. Stabilizing sensitive shales with inhibited potassium based drilling fluids. J Pet Technol. 1973;25(9):1089–100. https://doi.org/10.2118/4232-PA.
Osuji CE, Chenevert ME, Sharma MM. Effect of porosity and permeability on the membrane efficiency of shales. In: SPE annual technical conference, 21–24 September, Colorado, USA. 2008. https://doi.org/10.2118/116306-MS.
Parvizi Ghaleh S, Khodapanah E, Tabatabaei-Nezhad SAR. Comprehensive monolayer two-parameter isotherm and kinetic studies of thiamine adsorption on clay minerals: experimental and modeling approaches. J Mol Liq. 2020. https://doi.org/10.1016/j.molliq.2020.112942.
Patel AD. Design and development of quaternary amine compounds: shale inhibition with improved environmental profile. In: SPE international symposium on oilfield chemistry, 20–22 April, Woodlands, TX, USA. 2009. https://doi.org/10.2118/121737-MS.
Patel AD, Stamatakis S, Young S, Friedheim J. Advances in inhibitive water based drilling fluids—can they replace oil-based muds? In: SPE international symposium on oilfield chemistry. 28 February–2 March, Houston, TX, USA. 2007. https://doi.org/10.2118/106476-MS.
Pattison M, McMullin P, Bradbury JM, Alexander D, editors. Poultry diseases. Amsterdam: Elsevier Health Sciences; 2007. p. 520.
Reedy JH. Lecture demonstration of ammonium amalgam. J Chem Educ. 1929;10:1767. https://doi.org/10.1021/ed006p1767.
Riley M, Young S, Stamatakis E, Guo Q, Ji L, De Stefano G, Price K, Friedheim J. Wellbore stability in unconventional shales-the design of a nano-particle fluid. In: SPE oil and gas India conference and exhibition, 28–30 March, Mumbai, India. 2012. https://doi.org/10.2118/153729-MS.
Ritz M, Vaculikova L, Plevová E. Application of infrared spectroscopy and chemometric methods to identification of selected minerals. Acta Geodyn Geomater. 2011;8:47–58.
Sensoy T, Chenevert ME, Sharma MM. Minimizing water invasion in shales using nanoparticles. In: SPE annual technical conference and exhibition, 4–7 October, New Orleans, Louisiana, USA. 2009. https://doi.org/10.2118/124429-MS.
Shadizadeh SR, Moslemizadeh A, Dezaki AS. A novel nonionic surfactant for inhibiting shale hydration. Appl Clay Sci. 2015;118:74–86. https://doi.org/10.1016/j.clay.2015.09.006.
Sharma MM, Chenevert ME, Guo Q, Ji L, Friedheim J, Zhang R. A new family of nanoparticle based drilling fluids. In: SPE annual technical conference and exhibition, 8–10 October, San Antonio, TX, USA. 2012. https://doi.org/10.2118/160045-MS.
Sun L, Tanskanen JT, Hirvi JT, Kasa S, Schatz T, Pakkanen TA. Molecular dynamics study of montmorillonite crystalline swelling: roles of interlayer cation species and water content. Chem Phys. 2015;455:23–31. https://doi.org/10.1016/j.chemphys.2015.04.005.
Tamamura S, Takada T, Tomita J, Nagao S, Fukushi K, Yamamoto M. Salinity dependence of 226Ra adsorption on montmorillonite and kaolinite. J Radioanal Nucl Chem. 2014;299:569–75. https://doi.org/10.1007/s10967-013-2740-3.
Tan CP, Richards BG, Rahman S. Managing physico-chemical wellbore instability in shales with the chemical potential mechanism. In: SPE Asia Pacific oil and gas conference, 28–31 October, Adelaide, Australia. 1996. https://doi.org/10.2118/36971-MS.
van Oort E. On the physical and chemical stability of shales. J Pet Sci Eng. 2003;38(3–4):213–35. https://doi.org/10.1016/S0920-4105(03)00034-2.
Wang L, Liu S, Wang T, Sun D. Effect of poly (oxypropylene) diamine adsorption on hydration and dispersion of montmorillonite particles in aqueous solution. Colloids Surf A Physicochem Eng Asp. 2011;381(1–3):41–7. https://doi.org/10.1016/j.colsurfa.2011.03.008.
Xuan Y, Jiang G, Li Y, Wang J, Geng H. Inhibiting effect of dopamine adsorption and polymerization on hydrated swelling of montmorillonite. Colloids Surf A Physicochem Eng Asp. 2013;422:50–60. https://doi.org/10.1016/j.colsurfa.2013.01.038.
Zeynali ME. Mechanical and physico-chemical aspects of wellbore stability during drilling operations. J Pet Sci Eng. 2012;82:120–4. https://doi.org/10.1016/j.petrol.2012.01.006.
Zhang L, Wu X, Sun Y, Cai J, Lyu S. Experimental study of the pomelo peel powder as novel shale inhibitor in water-based drilling fluids. Energy Explor Exploit. 2019;38(2):569–88. https://doi.org/10.1177/0144598719882147.
Zhong H, Qiu Z, Huang W, Xie B, Wang W. Bis(hexamethylene)triamine as potential shale inhibitor in water-based drilling fluid. Open Pet Eng J. 2013;6(1):49–56. https://doi.org/10.2174/1874834101306010049.
Zhong H, Qiu Z, Huang W, Sun D, Zhang D, Cao J. Synergistic stabilization of shale by a mixture of polyamidoamine dendrimers modified bentonite with various generations in water-based drilling fluid. Appl Clay Sci. 2015;114:359–69. https://doi.org/10.1016/j.clay.2015.06.018.
Zhu X, Liu W. The effects of drill string impacts on wellbore stability. J Pet Sci Eng. 2013;109:217–29. https://doi.org/10.1016/j.petrol.2013.08.004.
Acknowledgements
The authors would like to appreciate Sahand Oil and Gas Research Institute (SOGRI) for providing the experimental facilities to conduct this research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parvizi Ghaleh, S., Khodapanah, E. & Tabatabaei-Nezhad, S.A. Experimental evaluation of thiamine as a new clay swelling inhibitor. Pet. Sci. 17, 1616–1633 (2020). https://doi.org/10.1007/s12182-020-00466-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-020-00466-6