Abstract
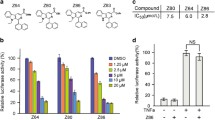
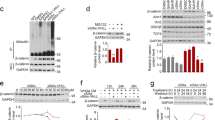
Aberrant β-catenin activation promotes the proliferation and survival of several types of tumor cells, including colorectal cancer (CRC) cells. Synthetic peptides are drug candidates for treating various diseases; however, peptide inhibitors of β-catenin have been rarely reported. A series of peptide inhibitors for β-catenin (F15A1–9k, F15A2–9k, and F15A3–9k) were designed and synthesized, and then used to treat human CRC cells (HT-29). Next, a series of in vitro assays, including cell counting, colony formation, flow cytometry, and Transwell assays, were performed to assess the biological effects of the peptides on CRC cells. Mouse xenograft models of HT-29 tumors were also used to evaluate the inhibitory effect of the peptide inhibitors on β-catenin expression in vivo. The inhibitory effect of the peptide inhibitors on β-catenin production was tested in a confocal laser scanning microscope study (CLSMS), and by H&E, TUNEL, and immunohistochemical (IHC) staining. The peptide inhibitors significantly reduced the viability of HT-29 cells in time- and concentration-dependent manners. Moreover, the peptide inhibitors for β-catenin significantly inhibited CRC tumorigenesis both in vitro and in vivo. Mechanistically, the peptide inhibitors for β-catenin inhibited the angiogenesis activity of HT-29 cells. When administered by itself, F15A2–9k blocked cell division, induced apoptosis, and reduced the migration and invasion capabilities of HT-29 cells, while a combination of F15A1–9k, F15A2–9k, and F15A3–9k showed even stronger inhibitory effects on HT-29 cells. In summary, the peptides designed to inhibit β-catenin demonstrated anti-tumor activity both in vitro and in vivo, suggesting their potential as therapeutic agents for treating CRC.






Similar content being viewed by others
References
Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H (1994) Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 107:3655–3663
Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13(1):11–26
Ayadi M, Bouygues A, Ouaret D, Ferrand N, Chouaib S, Thiery JP, Muchardt C, Sabbah M, Larsen AK (2015) Chronic chemotherapeutic stress promotes evolution of stemness and WNT/beta-catenin signaling in colorectal cancer cells: implications for clinical use of WNT-signaling inhibitors. Oncotarget 6(21):18518–18533
Barth AI, Nathke IS, Nelson WJ (1997) Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 9(5):683–6890
Chai Y, Xiang K, Wu Y, Zhang T, Liu Y, Liu X, Zhen W, Si Y (2018) Cucurbitacin B inhibits the Hippo-YAP signaling pathway and exerts anticancer activity in colorectal cancer cells. Med Sci Monitor 19(24):9251–9258
Di L (2015) Strategic approaches to optimizing peptide ADME properties. AAPS J 17(1):134–143
Dullius A, Rocha CM, Laufer S, de Souza CFV, Goettert MI (2019) Are peptides a solution for the treatment of hyperactivated JAK3 pathways? Inflammopharmacology 27(3):433–452
He T-C, Sparks AB, Rago C, Hermeking H, Zawel L, Costa LTD, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281(5382):1509–1512
Henninot A, Collins JC, Nuss JM (2018) The current state of peptide drug discovery: back to the future? J Med Chem 61(4):1382–1414
Ho SK, Thike AA, Cheok PY, Tse GM, Tan PH (2013) Phyllodes tumours of the breast: the role of CD34, vascular endothelial growth factor and beta-catenin in histological grading and clinical outcome. Histopathology 63(3):393–406
Jafary F, Ganjalikhany MR, Moradi A, Hemati M, Jafari S (2019) Novel peptide inhibitors for lactate dehydrogenase A (LDHA): a survey to inhibit LDHA activity via disruption of protein-protein interaction. Scientific reports 9(1):4686
Jiang J, Li Y, Jiang Z (2019) Effects of LDOC1 on colorectal cancer cells via downregulation of the Wnt/beta-catenin signaling pathway. Oncol Rep 41(6):3281–3291
Kaspar AA, Reichert JM (2013) Future directions for peptide therapeutics development. Drug Discov Today 18(17–18):807–817
Kekelidze M, D’Errico L, Pansini M, Tyndall A, Hohmann J (2013) Colorectal cancer: current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J Gastroenterol 19(46):8502–8514
Lancet T (2014) Toward better control of colorectal cancer. Lancet 383(9927):1437
Lipok M, Szlachcic A, Kindela K, Czyrek A, Otlewski J (2019) Identification of a peptide antagonist of the FGF1-FGFR1 signaling axis by phage display selection. FEBS Open Bio 9(5):914–924
Ma W, Li X, Song P, Zhang Q, Wu Z, Wang J, Li X, Xu R, Zhao W, Liu Y, Liu H, Yao X, Tang X, Chen P (2019) A vanillin derivative suppresses the growth of HT29 cells through the Wnt/beta-catenin signaling pathway. Eur J Pharmacol 15(849):43–49
Mook RA Jr, Wang J, Ren XR, Piao H, Lyerly HK, Chen W (2019) Identification of novel triazole inhibitors of Wnt/beta-catenin signaling based on the Niclosamide chemotype. Bioorg Med Chem Lett 29(2):317–321
Orford K, Orford CC, Byers SW (1999) Exogenous expression of beta-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol 146(4):855–867
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbiehurder A, Peter L (2017) An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547(7662):217–221
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272(5264):1023–1026
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain S, Masiarz F, Munemitsu S, Polakis PJS (1993) Association of the APC gene product with beta-catenin. Science 262(5140):1731–1734
Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, Woolfenden S, Yu KK, Markovits J, Killary K, Sonkin D, Yao YM, Warmuth M, Sellers WR, Schlegel R, Stegmeier F, Mosher RE, McLaughlin ME (2011) Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108(41):17135–17140
Serafino A, Moroni N, Psaila R, Zonfrillo M, Andreola F, Wannenes F, Mercuri L, Rasi G, Pierimarchi P (2012) Anti-proliferative effect of atrial natriuretic peptide on colorectal cancer cells: evidence for an Akt-mediated cross-talk between NHE-1 activity and Wnt/beta-catenin signaling. Biochim Biophys Acta 1822(6):1004–1018
Sun CQ, Arnold RS, Hsieh CL, Dorin JR, Lian F, Li Z, Petros JA (2019) Discovery and mechanisms of host defense to oncogenesis: targeting the beta-defensin-1 peptide as a natural tumor inhibitor. Cancer Biol Therapy 20:1–13
Sun H, Jiang C, Cong L, Wu N, Wang X, Hao M, Liu T, Wang L, Liu Y, Cong X (2018) CYP24A1 inhibition facilitates the antiproliferative effect of 1,25(OH)2D3 through downregulation of the WNT/beta-catenin pathway and methylation-mediated regulation of CYP24A1 in colorectal cancer cells. DNA Cell Biol 37(9):742–749
Thundimadathil J (2012) Cancer treatment using peptides: current therapies and future prospects. J Amino Acids 2012:967347
Vollmer RT (2016) A review of outcomes for colorectal adenomas. Am J Clin Pathol 146(5):567–571
Worni M, Shah KN, Clary BM (2014) Colorectal cancer with potentially resectable hepatic metastases: optimizing treatment. Curr Oncol Rep 16(10):407
Xin H, Wang C, Liu Z (2019) miR-196a-5p promotes metastasis of colorectal cancer via targeting IκBα. BMC Cancer 19(1):30
Zeng G, Apte U, Cieply B, Singh S, Monga SP (2007) siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia 9(11):951–959
Acknowledgements
This study was supported by grant from Basic Medical College of Shanxi Medical University (No. 20-1207).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Research Involving Animals Rights
All animal experiments were carried out strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Liu, X., Li, Y. et al. Novel Peptide Inhibitors of β-Catenin Effectively Suppress the Tumorigenesis of Colorectal Cancer. Int J Pept Res Ther 27, 263–274 (2021). https://doi.org/10.1007/s10989-020-10080-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10080-0