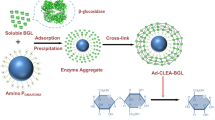
Abstract
Converting renewable cellulose into glucose via cellulase catalysis for further production of biofuel has been recognized as one of the most promising ways for solving energy crisis. However, the hydrolysis performance of immobilized cellulase was not satisfactory for practical application due to the reduced catalytic efficiency and lack of β-glucosidase (BG) component in cellulase. Here, a facile method was developed to sequentially co-immobilize BG and cellulase by polymeric microparticles with hierarchical structure. In this strategy, BG was firstly entrapped into the cross-linked poly(ethylene glycol) (PEG) microparticles via inverse emulsion polymerization initiated by isopropyl thioxanthone (ITX) under the irradiation of visible light, leaving the formed ITX semi-pinacol (ITXSP) dormant groups on surface of BG-loaded microparticles, which could be further activated by visible light irradiation and initiated a graft polymerization to introduce poly(acrylic acid) (PAA) brush on the PEG core. After that, cellulase was covalently bonded on the PAA chains via carbodiimide reaction. The synergic effect of BG and cellulase was verified in the dual enzyme immobilization system, which led to a better stability at a wide range of temperature and pH than free enzymes. The dual enzymes system exhibited excellent reusability, which could retain 75% and 57% of the initial activity after 10 cycles of hydrolysis of carboxyl methyl cellulose and 5 cycles of hydrolysis of filter paper, respectively, indicative of the potential in biofuel areas in a cost-effective manner.
Similar content being viewed by others
References
Shen, X. J.; Wen, J. L.; Mei, Q. Q.; Chen, X.; Sun, D.; Yuan, T. Q.; Sun, R. C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem.2019, 21, 275–283.
Alonso, D. M.; Bond, J. Q.; Dumesic, J. A. Catalytic conversion of biomass to biofuels. Green Chem.2010, 12, 1493–1513.
Bugg, T. D. H.; Rahmanpour, R. Enzymatic conversion of lignin into renewable chemicals. Curr. Opin. Chem. Biol.2015, 29, 10–17.
Dutta, S.; Wu, K. C. W. Enzymatic breakdown of biomass: enzyme active sites, immobilization, and biofuel production. Green Chem.2014, 16, 4615–4626.
Gupta, V. K.; Kubicek, C. P.; Berrin, J. G.; Wilson, D. W.; Couturier, M.; Berlin, A.; Filho, E. X. F.; Ezeji, T. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci.2016, 41, 633–645.
Srivastava, N.; Srivastava, M.; Mishra, P. K.; Gupta, V. K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P. W. Applications of fungal cellulases in biofuel production: advances and limitations. Renew. Sust. Energ. Rev.2018, 82, 2379–2386.
Peng, H.; Rübsam, K.; Jakob, F.; Schwaneberg, U.; Pich, A. Tunable enzymatic activity and enhanced stability of cellulase immobilized in biohybrid nanogels. Biomacromolecules2016, 17, 3619–3631.
Shanmugam, S.; Ngo, H. H.; Wu, Y. R. Advanced CRISPR/Cas-based genome editing tools for microbial biofuels production: a review. Renew. Energ.2020, 149, 1107–1119.
Payne, C. M.; Knott, B. C.; Mayes, H. B.; Hansson, H.; Himmel, M. E.; Sandgren, M.; Ståhlberg, J.; Beckham, G. T. Fungal cellulases. Chem. Rev.2015, 115, 1308–1448.
Rodrigues, R. C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev.2013, 42, 6290–6307.
Mackenzie, K. J.; Francis, M. B. Recyclable thermoresponsive polymer-cellulase bioconjugates for biomass depolymerization. J. Am. Chem. Soc.2013, 135, 293–300.
Han, J.; Wan, J.; Wang, Y.; Wang, L.; Li, C.; Mao, Y.; Ni, L. Recyclable soluble-insoluble upper critical solution temperature-type poly(methacrylamide-co-acrylic acid)-cellulase biocatalyst for hydrolysis of cellulose into glucose. ACS Sustain. Chem. Eng.2018, 6, 7779–7788.
Saini, J. K.; Patel, A. K.; Adsul, M.; Singhania, R. R. Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renew. Energ.2016, 98, 29–42.
Tully, J.; Yendluri, R.; Lvov, Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules2016, 17, 615–621.
Kamat, R. K.; Zhang, Y.; Anuganti, M.; Ma, W.; Noshadi, I.; Fu, H.; Ekatan, S.; Parnas, R.; Wang, C.; Kumar, C. V.; Lin, Y. Enzymatic activities of polycatalytic complexes with nonprocessive cellulases immobilized on the surface of magnetic nanoparticles. Langmuir2016, 32, 11573–11579.
Qi, B.; Luo, J.; Wan, Y. Immobilization of cellulase on a core-shell structured metal-organic framework composites: better inhibitors tolerance and easier recycling. Bioresour. Technol.2018, 268, 577–582.
Cho, E. J.; Jung, S.; Kim, H. J.; Lee, Y. G.; Nam, K. C.; Lee, H. J.; Bae, H. J. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun.2012, 48, 886–888.
Limadinata, P. A.; Li, A.; Li, Z. Temperature-responsive nanobiocatalysts with an upper critical solution temperature for high performance biotransformation and easy catalyst recycling: efficient hydrolysis of cellulose to glucose. Green Chem.2015, 17, 1194–1203.
Ng, I. S.; Tsai, S. W.; Ju, Y. M.; Yu, S. M.; Ho, T. D. Dynamic synergistic effect on Trichoderma reesei cellulases by novel β-glucosidases from Taiwanese fungi. Bioresour. Technol.2011, 102, 6073–6081.
Chávez-Guerrero, L.; Silva-Mendoza, J.; Sepúlveda-Guzmán, S.; Medina-Aguirre, N. A.; Vazquez-Rodriguez, S.; Cantú-Cárdenas, M. E.; García-Gómez, N. A. Enzymatic hydrolysis of cellulose nanoplatelets as a source of sugars with the concomitant production of cellulose nanofibrils. Carbohyd. Polym.2019, 210, 85–91.
Hsieh, C. W. C.; Cannella, D.; Jørgensen, H.; Felby, C.; Thygesen, L. G. Cellulase inhibition by high concentrations of monosaccharides. J. Agric. Food. Chem.2014, 62, 3800–3805.
Teugjas, H.; Väljamäe, P. Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol. Biofuels2013, 6, 105.
Borges, D. G.; Baraldo Junior, A.; Farinas, C. S.; de Lima Camargo Giordano, R.; Tardioli, P. W. Enhanced saccharification of sugarcane bagasse using soluble cellulase supplemented with immobilized β-glucosidase. Bioresour. Technol.2014, 167, 206–213.
Pallapolu, V. R.; Lee, Y. Y.; Garlock, R. J.; Balan, V.; Dale, B. E.; Kim, Y.; Mosier, N. S.; Ladisch, M. R.; Falls, M.; Holtzapple, M. T.; Sierra-Ramirez, R.; Shi, J.; Ebrik, M. A.; Redmond, T.; Yang, B.; Wyman, C. E.; Donohoe, B. S.; Vinzant, T. B.; Elander, R. T.; Hames, B.; Thomas, S.; Warner, R. E. Effects of enzyme loading and β-glucosidase supplementation on enzymatic hydrolysis of switchgrass processed by leading pretreatment technologies. Bioresour. Technol.2011, 102, 11115–11120.
Liu, J.; Cao, X. Biodegradation of cellulose by β-glucosidase and cellulase immobilized on a pH-responsive copolymer. Biotechnol. Bioproc. E.2014, 19, 829–837.
Chakrabarti, A. C.; Storey, K. B. Enhanced glucose production from cellulose using coimmobilized cellulase and β-glucosidase. Appl. Biochem. Biotech.1989, 22, 263–278.
Storey, K. B.; Chakrabarti, A. C. One-step conversion of cellulose to fructose using coimmobilized cellulase, β-glucosidase, and glucose isomerase. Appl. Biochem. Biotech.1990, 23, 139–154.
Wang, Y.; Qi, Y.; Chen, C.; Zhao, C.; Ma, Y.; Yang, W. Layered coimmobilization of β-glucosidase and cellulase on polymer film by visible-light-induced graft polymerization. ACS Appl. Mater. Interfaces2019, 47, 44913–44921.
Figueira, J. A.; Sato, H. L. H.; Fernandes, P. Establishing the feasibility of using β-glucosidase entrapped in Lentikats and in sol-gel supports for cellobiose hydrolysis. J. Agric. Food. Chem.2013, 61, 626–634.
Tu, M.; Zhang, X.; Kurabi, A.; Gilkes, N.; Mabee, W.; Saddler, J. Immobilization of β-glucosidase on Eupergit C for lignocellulose hydrolysis. Biotechnol. Lett.2006, 28, 151–156.
Javed, M. R.; Buthe, A.; Rashid, M. H.; Wang, P. Cost-efficient entrapment of β-glucosidase in nanoscale latex and silicone polymeric thin films for use as stable biocatalysts. Food Chem.2016, 190, 1078–1085.
Wang, G.; Chen, D.; Zhang, L.; Wang, Y.; Zhao, C.; Yan, X.; He, B.; Ma, Y.; Yang, W. A mild route to entrap papain into cross-linked PEG microparticles via visible light-induced inverse emulsion polymerization. J. Mater. Sci.2018, 53, 880–891.
Chang, R. H. Y.; Jang, J.; Wu, K. C. W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem.2011, 13, 2844–2850.
Zhang, Y.; Xu, J.; Yuan, Z.; Xu, H.; Yu, Q. Artificial neural network-genetic algorithm based optimization for the immobilization of cellulase on the smart polymer Eudragit L-100. Bioresour. Technol.2010, 101, 3153–3158.
Ghose, T. Measurement of cellulase activities. Pure Appl. Chem.1987, 59, 257–268.
Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res.2014, 53, 3448–3454.
Yang, P.; Yang, W. Surface chemoselective phototransformation of C―H bonds on organic polymeric materials and related hightech applications. Chem. Rev.2013, 113, 5547–5594.
Wang, G.; Zhang, K.; Wang, Y.; Zhao, C.; He, B.; Ma, Y.; Yang, W. Decorating an individual living cell with a shell of controllable thickness by cytocompatible surface initiated graft polymerization. Chem. Commun.2018, 54, 4677–4680.
He, B.; Zhu, X.; Zhao, C.; Ma, Y.; Yang, W. Sequential coimmobilization of β-glucosidase and yeast cells on single polymer support for bioethanol production. Sci. China Chem.2018, 61, 1600–1608.
Burugapalli, K.; Bhatia, D.; Koul, V.; Choudhary, V. Interpenetrating polymer networks based on poly(acrylic acid) and gelatin. I: Swelling and thermal behavior. J. Appl. Polym. Sci.2001, 82, 217–227.
Cheng, Z.; Wu, C.; Yang, W.; Xu, T. Preparation of bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) hollow fiber cation-exchange membranes and immobilization of cellulase thereon. J. Membr. Sci.2012, 358, 93–100.
Zhu, X.; Ma, Y.; Zhao, C.; Lin, Z.; Zhang, L.; Chen, R.; Yang, W. A mild strategy to encapsulate enzyme into hydrogel layer grafted on polymeric substrate. Langmuir2014, 30, 15229–15237.
Romano, E. J.; Schulz, K. H. A XPS investigation of SO2 adsorption on ceria-zirconia mixed-metal oxides. Appl. Surf. Sci.2005, 246, 262–270.
Chen, Y.; Gao, Y.; da Silva, L. P.; Pirraco, R. P.; Ma, M.; Yang, L.; Reis, R. L.; Chen, J. A thermo-/pH-responsive hydrogel (PNIPAM-PDMA-PAA) with diverse nanostructures and gel behaviors as a general drug carrier for drug release. Polym. Chem.2018, 9, 4063–4072.
Aulich, D.; Hoy, O.; Luzinov, I.; Eichhorn, K. J.; Stamm, M.; Gensch, M.; Schade, U.; Esser, N.; Hinrichs, K. In-situ IR synchrotron mapping ellipsometry on stimuli-responsive PAA-b-PS/PEG mixed polymer brushes. Phys. Status Solidi C2010, 7, 197–199.
Xu, W.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. Immobilization of cellulase proteins on zeolitic imidazolate framework (ZIF-8)/polyvinylidene fluoride hybrid membranes. New J. Chem.2018, 42, 17429–17438.
Liu, X.; Fang, Y.; Yang, X.; Li, Y.; Wang, C. Electrospun nanofibrous membranes containing epoxy groups and hydrophilic polyethylene oxide chain for highly active and stable covalent immobilization of lipase. Chem. Eng. J.2018, 336, 456–464.
Gokhale, A. A.; Lu, J.; Lee, I. Immobilization of cellulase on magnetoresponsive graphene nano-supports. J. Mol. Catal. B: Enzym.2013, 90, 76–86.
Li, C.; Yoshimoto, M.; Fukunaga, K.; Nakao, K. Characterization and immobilization of liposome-bound cellulase for hydrolysis of insoluble cellulose. Bioresour. Technol.2007, 98, 1366–1372.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51873014, 51521062, and 51473015).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Information
10118_2020_2437_MOESM1_ESM.pdf
Facile Construction of Synergistic β-Glucosidase and Cellulase Sequential Co-immobilization System for Enhanced Biomass Conversion
Rights and permissions
About this article
Cite this article
Wang, G., Zhang, K., Xin, JY. et al. Facile Construction of Synergistic β-Glucosidase and Cellulase Sequential Co-immobilization System for Enhanced Biomass Conversion. Chin J Polym Sci 38, 1277–1285 (2020). https://doi.org/10.1007/s10118-020-2437-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-020-2437-3