Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction
Abstract
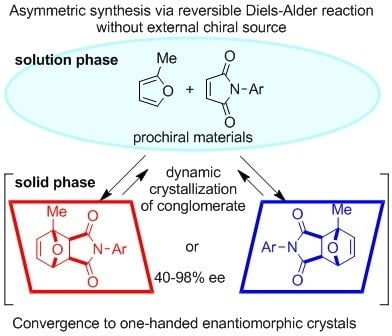
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Diels, O.; Alder, K. Synthesen in der Hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der Hydroaromatischen Reihe, IV. Mitteilung: Über die Anlagerung von Maleinsäure-anhydrid an Arylierte Diene, Triene und Fulvene. Ber. Dtsch. Chem. Ges. 1929, 62, 2081–2087. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Takao, K.-I.; Munakata, R.; Tadano, K. Recent advances in natural product synthesis by using intramolecular Diels-Alder reactions. Chem. Rev. 2005, 105, 4779–4807. [Google Scholar] [CrossRef]
- Min, L.; Liu, X.; Li, C.-C. Total Synthesis of Natural Products with Bridged Bicyclo[m.n.1] Ring Systems via Type II [5 + 2] Cycloaddition. Acc. Chem. Res. 2020, 53, 703–718. [Google Scholar] [CrossRef]
- Breunig, M.; Yuan, P.; Gaich, T. An Unexpected Transannular [4 + 2] Cycloaddition during the Total Synthesis of (+)-Norcembrene. Angew. Chem. Int. Ed. 2020, 59, 5521–5525. [Google Scholar] [CrossRef]
- Chavan, S.P.; Kadam, A.L.; Gonnade, R.G. Enantioselective Formal Total Synthesis of (-)-Quinagolide. Org. Lett. 2019, 21, 9089–9093. [Google Scholar] [CrossRef]
- Burns, A.S.; Rychnovsky, S.D. Total Synthesis and Structure Revision of (-)-Illisimonin A, a Neuroprotective Sesquiterpenoid from the Fruits of Illicium simonsii. J. Am. Chem. Soc. 2019, 141, 13295–13300. [Google Scholar] [CrossRef]
- Zhou, S.; Xia, K.; Leng, X.; Li, A. Asymmetric Total Synthesis of Arcutinidine, Arcutinine, and Arcutine. J. Am. Chem. Soc. 2019, 141, 13718–13723. [Google Scholar] [CrossRef]
- Maurya, V.; Appayee, C. Enantioselective Total Synthesis of Potent 9β-11-Hydroxyhexahydrocannabinol. J. Org. Chem. 2020, 85, 1291–1297. [Google Scholar] [CrossRef]
- Corey, E.J.; Shibata, T.; Lee, T.W. Asymmetric Diels-Alder Reactions Catalyzed by a Triflic Acid Activated Chiral Oxazaborolidine. J. Am. Chem. Soc. 2002, 124, 3808–3809. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.; Reed, L.A.; Masamune, S. Asymmetric Diels-Alder Reaction: Design of Chiral Dienophiles. J. Org. Chem. 1983, 48, 1137–1139. [Google Scholar] [CrossRef]
- Oppolzer, W. Asymmetric Diels-Alder and Ene Reactions in Organic Synthesis. New Synthetic Methods (48). Angew. Chem. Int. Ed. 1984, 23, 876–889. [Google Scholar] [CrossRef]
- Kagan, H.B.; Riant, O. Catalytic Asymmetric Diels-Alder Reactions. Chem. Rev. 1992, 92, 1007–1019. [Google Scholar] [CrossRef]
- Mehta, G.; Uma, R. Stereoelectronic Control in Diels−Alder Reaction of Dissymmetric 1,3-Dienes. Acc. Chem. Res. 2000, 33, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Jen, W.S.; MacMillan, D.W.C. Enantioselective Organocatalytic Intramolecular Diels−Alder Reactions. The Asymmetric Synthesis of Solanapyrone D. J. Am. Chem. Soc. 2005, 127, 11616–11617. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lin, L.; Liu, X.; Hu, X.; Lu, Y.; Zhang, X.; Feng, X. Catalytic Asymmetric Diels-Alder Reaction/[3,3] Sigmatropic Rearrangement Cascade of 1-Thiocyanatobutadienes. Angew. Chem. Int. Ed. 2018, 57, 9113–9116. [Google Scholar] [CrossRef]
- Li, M.; Carreras, V.; Jalba, A.; Ollevier, T. Asymmetric Diels-Alder Reaction of α,β-Unsaturated Oxazolidin-2-one Derivatives Catalyzed by a Chiral Fe(III)-Bipyridine Diol Complex. Org. Lett. 2018, 20, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Lin, L.; Fu, K.; Zheng, H.; Liu, X.; Feng, X. Synthesis of Optically Pure Spiro[cyclohexane-oxindoline] Derivatives via Catalytic Asymmetric Diels-Alder Reaction of Brassard-Type Diene with Methyleneindolines. J. Org. Chem. 2015, 80, 8836–8842. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kumar, P.; Singh, S. Synthesis of MacMillan catalyst modified with ionic liquid as a recoverable catalyst for asymmetric Diels-Alder reaction. RSC Adv. 2015, 5, 52636–52641. [Google Scholar] [CrossRef]
- Walborsky, H.; Barash, L.; Davis, T. Communications- Partial Asymmetric Syntheses: The Diels-Alder Reaction. J. Am. Chem. Soc. 1988, 110, 1238–1256. [Google Scholar]
- Evans, D.A.; Chapman, K.T.; Bisaha, J. Asymmetric Diels-Alder Cycloaddition Reactions with Chiral α,β-Unsaturated N-Acyloxazolidinones. J. Am. Chem. Soc. 1988, 110, 1238–1256. [Google Scholar] [CrossRef]
- Robiette, R.; Cheboub-Benchaba, K.; Peeters, D.; Marchand-Brynaert, J. Design of a New and Highly Effective Chiral Auxiliary for Diels-Alder Reaction of 1-Aminodiene. J. Org. Chem. 2003, 68, 9809–9812. [Google Scholar] [CrossRef] [PubMed]
- Lakner, F.J.; Negrete, G.R. A new and convenient chiral auxiliary for asymmetric Diels-Alder cycloadditions in environmentally benign solvents. Synlett 2002, 4, 643–645. [Google Scholar] [CrossRef]
- Hachiya, S.; Kasashima, Y.; Yagishita, F.; Mino, T.; Masu, H.; Sakamoto, M. Asymmetric Transformation by Dynamic Crystallization of Achiral Succinimides. Chem. Commun. 2013, 49, 4776–4778. [Google Scholar] [CrossRef]
- Addadi, L.; Lahav, M. Origin of Optical Activity in Nature; Walker, D.C., Ed.; Elsevier: New York, NY, USA, 1979. [Google Scholar]
- Mason, S.F. Origins of Biomolecular Handedness. Nature 1984, 311, 19–23. [Google Scholar] [CrossRef]
- Bonner, W.A. The Origin and Amplification of Biomolecular Chirality. Orig. Life Evol. Biosph. 1991, 21, 59. [Google Scholar] [CrossRef]
- Avalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C.; Barron, L.D. Absolute Asymmetric Synthesis under Physical Fields: Facts and Fictions. Chem. Rev. 1998, 98, 2391–2404. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R. Absolute Asymmetric Synthesis: The Origin, Control, and Amplification of Chirality. Angew. Chem. Int. Ed. 1999, 38, 3418–3438. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Wei, S.; Freund, M.; Mauksch, M. Deracemization with reversible Mannich type reaction. Angew. Chem. Int. Ed. 2009, 48, 590–594. [Google Scholar] [CrossRef]
- Flock, A.M.; Reucher, C.M.M.; Bolm, C. Enantioenrichment by Iterative Retro-Aldol/Aldol ReactionCatalyzed by an Achiral or Racemic Base. Chem. Eur. J. 2010, 16, 3918–3921. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, F.; Ishikawa, H.; Onuki, T.; Hachiya, S.; Mino, T.; Sakamoto, M. Total spontaneous resolution by deracemization of isoindolinones. Angew. Chem. Int. Ed. 2012, 51, 13023–13025. [Google Scholar] [CrossRef] [PubMed]
- Steendam, R.R.E.; Verkade, J.M.M.; Van Benthem, T.J.B.; Meekes, H.; Van Enckevort, W.J.P.; Raap, J.; Rutjes, F.P.J.T.; Vlieg, E. Emergence of Single-molecular Chirality from Achiral Reactants. Nat. Commun. 2014, 5, 5543. [Google Scholar]
- Kaji, Y.; Uemura, N.; Kasashima, Y.; Ishikawa, H.; Yoshida, Y.; Mino, T.; Sakamoto, M. Asymmetric Synthesis of an Amino Acid Derivative from Achiral Aroyl Acrylamide by Reversible Michael Addition and Preferential Crystallization. Chem. Eur. J. 2016, 22, 16429–16432. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Sano, K.; Matsumoto, A.; Yoshida, Y.; Mino, T.; Sakamoto, M. Absolute Asymmetric Synthesis of an Aspartic Acid Derivative from Prochiral Maleic Acid and Pyridine under Achiral Conditions. Chem. Asian J. 2019, 14, 4150–4153. [Google Scholar] [CrossRef]
- Kawasaki, T.; Takamatsu, N.; Aiba, S.; Tokunaga, Y. Spontaneous Formation and Amplification of an Enantioenriched α-Amino Nitrile: A Chiral Precursor for Strecker Amino Acid Synthesis. Chem. Commun. 2015, 51, 14377–14380. [Google Scholar] [CrossRef]
- Takamatsu, N.; Aiba, S.; Yamada, T.; Tokunaga, Y.; Kawasaki, T. Highly Stereoselective Strecker Synthesis Induced by a Slight Modification of Benzhydrylamine from Achiral to Chiral. Chem. Eur. J. 2018, 24, 1304–1310. [Google Scholar] [CrossRef]
- Sakamoto, M.; Shiratsuki, K.; Uemura, N.; Ishikawa, H.; Yoshida, Y.; Kasashima, Y.; Mino, T. Asymmetric Synthesis by Using Natural Sunlight under Absolute Achiral Conditions. Chem. Eur. J. 2017, 23, 1717–1721. [Google Scholar] [CrossRef]
- Ishikawa, H.; Uemura, N.; Yagishita, Y.; Baba, N.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Asymmetric Synthesis Involving Reversible Photodimerization of a Prochiral Flavonoid Followed by Crystallization. Eur. J. Org. Chem. 2017, 46, 6878–6881. [Google Scholar] [CrossRef]
- Uemura, N.; Toyoda, S.; Ishikawa, H.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Asymmetric Diels−Alder Reaction Involving Dynamic Enantioselective Crystallization. J. Org. Chem. 2018, 83, 9300–9304. [Google Scholar] [CrossRef]
- Viedma, C. Chiral Symmetry Breaking during Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquerel, G. Crystallization of Molecular Systems from Solution: Phase Diagrams, Supersaturation and Other Basic Concepts. Chem. Soc. Rev. 2014, 43, 2286–2300. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C.; Ortiz, J.E.; de Torres, T.; Izumi, T.; Blackmond, D.G.; Viedma, C.; Ortiz, J.E.; de Torres, T.; Izumi, T.; Blackmond, D.G. Evolution of Solid Phase Homochirality for a Proteinogenic Amino Acid. J. Am. Chem. Soc. 2008, 130, 15274–15275. [Google Scholar] [CrossRef] [PubMed]
- Noorduin, W.L.; Bode, A.A.C.; van der Meiden, M.; Meekes, H.; van Etteger, A.F.; van Enckevort, W.J.P.; Christianen, P.C.M.; Kaptein, B.; Kellogg, R.M.; Rasing, T.; et al. Complete Chiral Symmetry Breaking of an Amino Acid Derivative Directed by Circularly Polarized Light. Nature Chem. 2009, 1, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Gherase, D.; Conroy, D.; Matar, O.K.; Blackmond, D.G. Experimental and Theoretical Study of the Emergence of Single Chirality in Attrition-Enhanced Deracemization. Cryst. Growth Des. 2014, 14, 928–937. [Google Scholar] [CrossRef]
- Sogutoglu, L.-C.; Steendam, R.R.E.; Meekes, H.; Vlieg, E.; Rutjes, F.P.J.T. Viedma Ripening: A Reliable Crystallisation Method to Reach Single Chirality. Chem. Soc. Rev. 2015, 44, 6723–6732. [Google Scholar] [CrossRef] [Green Version]
- Steendam, R.R.E.; Kulka, M.W.; Meekes, H.; van Enckevort, W.J.P.; Raap, J.; Vlieg, E.; Rutjes, F.P.J.T. One-Pot Synthesis, Crystallization and Deracemization of Isoindolinones from Achiral Reactants. Eur. J. Org. Chem. 2015, 7249–7252. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.T.; Cheung, P.S.M.; Werber, L.; Gagnon, J.; Sivakumar, R.; Lennox, C.; Sossin, A.; Mastai, Y.; Cuccia, L.A. Directing the Viedma ripening of ethylenediammonium sulfate using “Tailor-made” chiral additives. Chem. Commun. 2016, 52, 12626–12629. [Google Scholar] [CrossRef]
- Sivakumar, R.; Askari, M.S.; Woo, S.; Madwar, C.; Ottenwaelder, X.; Bohle, D.S.; Cuccia, L.A. Homochiral crystal generation via sequential dehydration and Viedma ripening. Cryst. Eng. Comm. 2016, 18, 4277–4280. [Google Scholar] [CrossRef]
- Breveglieri, F.; Maggioni, G.M.; Mazzotti, M. Deracemization of NMPA via Temperature Cycles. Cryst. Growth Des. 2018, 18, 1873–1881. [Google Scholar] [CrossRef] [Green Version]
- Engwerda, A.H.J.; Maassen, R.; Tinnemans, P.; Meekes, H.; Rutjes, F.P.J.T.; Vlieg, E. Attrition-Enhanced Deracemization of the Antimalaria Drug Mefloquine. Angew. Chem. Int. Ed. 2019, 58, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N.; Blackmond, D.G. Isotopically Directed Symmetry Breaking and Enantioenrichment in Attrition-Enhanced Deracemization. J. Am. Chem. Soc. 2020, 142, 3873–3879. [Google Scholar]
- Ishikawa, H.; Ban, K.; Uemura, N.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Attrition-Enhanced Deracemization of Axially Chiral Nicotinamides. Eur. J. Org. Chem. 2020, 8, 1001–1005. [Google Scholar] [CrossRef]
- Havinga, E. Spontaneous Formation of Optically Active Substances. Biochim. Biophys. Acta 1954, 13, 171–174. [Google Scholar] [CrossRef]
- Frank, F.C. On Spontaneous Asymmetric Synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Yoshioka, R. Racemization, Optical Resolution and Crystallization-induced Asymmetric Transformation of Amino Acids and Pharmaceutical Intermediates. Top. Curr. Chem. 2007, 269, 83–132. [Google Scholar]
- Sakamoto, M.; Mino, T. Asymmetric Reaction Using Molecular Chirality Controlled by Spontaneous Crystallization. Eur. J. Org. Chem. 2017, 6878–6881. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates and Resolution; Krieger: Malabar, FL, USA, 1994. [Google Scholar]
- Coquerel, G. Chiral Discrimination in the Solid State: Applications to Resolution and Deracemization; Springer: Tokyo, Japan, 2015; pp. 393–420. [Google Scholar]
- Kellogg, R.M. How to Use Pasteur’s Tweezers; Springer: Tokyo, Japan, 2015; pp. 421–443. [Google Scholar]
- Sakamoto, M.; Mino, T. Total Resolution of Racemates by Dynamic Preferential Crystallization; Springer: Tokyo, Japan, 2015; pp. 445–462. [Google Scholar]
- Petralli-Mallow, T.; Wong, T.M.; Byers, J.D.; Yee, H.I.; Hicks, J.M. Circular Dichroism Spectroscopy at Interfaces: A Surface Second Harmonic Generation Study. J. Phys. Chem. 1993, 97, 1383–1388. [Google Scholar] [CrossRef]
- Fischer, P.; Hache, F. Nonlinear Optical Spectroscopy of Chiral Molecules. Chirality 2005, 17, 421–437. [Google Scholar] [CrossRef]
- Kotha, S.; Banerjee, S. Recent developments in the retro-Diels–Alder reaction. RSC Adv. 2013, 3, 7642–7666. [Google Scholar] [CrossRef]
- Saito, Y.; Hyuga, H. Chiral Crystal Growth under Grinding. J. Phys. Soc. Jpn. 2008, 77, 113001/1. [Google Scholar] [CrossRef]
- Martin, I.; Marco, M.A. A Population Balance Model for Chiral Resolution via Viedma Ripening. Cryst. Growth Des. 2011, 11, 4611. [Google Scholar]
- Peter, J.S. Kinetics and Thermodynamics of Efficient Chiral Symmetry Breaking in Nearly Racemic Mixtures of Conglomerate Crystals. Cryst. Growth Des. 2011, 11, 1957–1965. [Google Scholar]
- Xiouras, C.; Van Cleemput, E.; Kumpen, A.; Ter Horst, J.H.; Van Gerven, T.; Stefanidis, G.D. Towards Deracemization in the Absence of Grinding through Crystal Transformation, Ripening, and Racemization. Cryst. Growth Des. 2017, 17, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Matuszak, N.; Muccioli, G.G.; Labar, G.; Lambert, D.M. Synthesis and in Vitro Evaluation of N-Substituted Maleimide Derivatives as Selective Monoglyceride Lipase Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1510–1515. [Google Scholar] [CrossRef]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent Nematicidal Activity of Maleimide Derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]







| exo-3 | R | Space Group |
|---|---|---|
| 3a | C6H5 | P212121[a] |
| 3b | 4-ClC6H4 | P212121, (73:27) [b] |
| 3c | 3,4-Me2C6H3 | P212121 |
| 3d | 3,5-Cl2C6H3 | P21, (76:24) [b] |
| 3e | 4-MeC6H4 | P212121, polymorphism [c] |
| 3f | 4-FC6H4 | P21/c |
| 3g | 4-BrC6H4 | P21/n |
| 3h | 3-MeC6H4 | ND [d] |
| 3i | 4-EtC6H4 | ND [d] |
| 3j | 4-MeOC6H4 | ND [d] |
| Substrate | τ1/2 of 2 (h) | τ1/2 of exo-3 (h) | ||
|---|---|---|---|---|
| DA w/o TFA [b] | DA with TFA [c] | retro-DA w/o TFA [d] | retro-DA with TFA [e] | |
| a[f] | 0.92 | 0.25 | 4.5 | 3.0 |
| b | 0.30 | <0.1 | 4.0 | 2.0 |
| c | 0.42 | <0.1 | 6.5 | 3.2 |
| d | 0.30 | <0.1 | 2.0 | 1.2 |
| 3 | Time (day) [b] | Yield (%) [c] | Ee (%) [d] |
|---|---|---|---|
| 3a[e] | 6–14 | 80 | 90 |
| 3b | 7–15 | 70 | 40 |
| 3c | 3–9 | 81 | 98 |
| 3d | 4–8 | 78 | 49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, N.; Toyoda, S.; Shimizu, W.; Yoshida, Y.; Mino, T.; Sakamoto, M. Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction. Symmetry 2020, 12, 910. https://doi.org/10.3390/sym12060910
Uemura N, Toyoda S, Shimizu W, Yoshida Y, Mino T, Sakamoto M. Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction. Symmetry. 2020; 12(6):910. https://doi.org/10.3390/sym12060910
Chicago/Turabian StyleUemura, Naohiro, Seiya Toyoda, Waku Shimizu, Yasushi Yoshida, Takashi Mino, and Masami Sakamoto. 2020. "Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels–Alder Reaction" Symmetry 12, no. 6: 910. https://doi.org/10.3390/sym12060910