Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study
Abstract
:1. Introduction
2. Theoretical Model
2.1. Mass Transfer
2.2. Heat Transfer
2.2.1. Heat Transfer from the Feed Side to the Membrane Surface
2.2.2. Heat Transfer through the Membrane Layer
2.2.3. Heat Transfer from the Membrane Surface to the Permeate Stream
3. Materials and Methods
3.1. Membrane and Membrane Module
3.2. Experimental Setup and Procedure
4. Results and Discussion
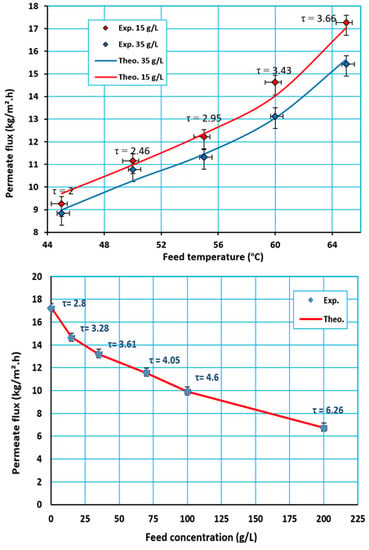
4.1. Effect of Hot Feed Temperature
4.2. Effect of Feed Concentration
4.3. Effect of the Feed Flow Rate
4.4. Theoretical Results
4.5. Verification of the Mathematical Model with Previous Works
4.6. Thermal Efficiency
4.7. Gain Output Ratio ()
4.8. Temperature Polarization Coefficient ()
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclatures\Capital Letters
| A | Effective Surface Area (m2) |
| Geometric factor | |
| C | Concentration (g·mol/m3) or (g/L) |
| Specific heat capacity (J/kg·K) | |
| Equivalent diffusion coefficient (kg/Pa·m2·s) | |
| Hydraulic diameter (m) | |
| Knudsen diffusion coefficient (kg/Pa·m2·s) | |
| Molecular diffusion coefficient (kg/Pa·m2·s) | |
| Water–air diffusivity (m2/s) | |
| Gain output ration | |
| Permeate mass flux (kg/m2·s) or (kg/m2·h) | |
| L | Effective membrane length (m) |
| Liquid entrance pressure (Pa) | |
| Permeate mass (kg) | |
| Molecular weight (g/g·mol) | |
| Heat flux (W/m2) | |
| Pressure (Pa) | |
| Vapor pressure (Pa) | |
| Universal gas constant (8.314 J/g·mol·K) | |
| T | Temperature (°C or K) |
| Mean temperature (°C or K) | |
| W | Effective membrane width (m) |
Lower Case Letters
| Pore Size (diameter) (m) | |
| h | Heat transfer coefficient (W/m2 K) |
| k | Thermal conductivity (W/m·K) |
| Solute mass transfer coefficient (m/s) | |
| m | Mass flow rate (kg/s) |
| Partial pressure (Pa) | |
| Maximum pore radius (m) | |
| t | Experiment time |
| u | Velocity (m/s) |
| Mole fraction |
Greek Letters
| ΔH | Latent Heat of Vaporization (kJ/kg) |
| Activity coefficient | |
| Surface tension (N/m) | |
| Membrane thickness (m) | |
| ε | Porosity |
| Thermal efficiency | |
| Contact angle (°) | |
| µ | Viscosity (Pa·s) |
| ρ | density (kg/m3) |
| Hypothesis path through the membrane (m) | |
| Tortuosity | |
| Concentration polarization coefficient |
Subscripts
| b | At the Bulk |
| C | Conducted |
| Feed side | |
| g | Gas |
| K | Knudson diffusion |
| M | Molecular diffusion |
| Membrane | |
| Permeate side | |
| s | Membrane solid |
| sol | Solution |
| v | Evaporative |
| w | Water |
Dimensionless Numbers
| Nusselt No. | |
| Prandtl No. | |
| Reynolds No. | |
| Schmidt No. | |
| Sherwood No. |
References
- Bahar, R. Conversion of Saline Water to Fresh Water Using Air Gap Membrane Distillation (AGMD). Ph.D. Thesis, University of Singapore, Singapore, 2010. [Google Scholar]
- Fadhil, S.; Alsalhy, Q.F.; Makki, H.; Figueroa, R.; Marino, T.; Criscuoli, A.; Macedonio, F.; Giorno, L.; Drioli, E.; Figoli, A. Seawater desalination using PVDF-HFP membrane in DCMD process: Assessment of operating condition by response surface method. Chem. Eng. Commun. 2019, 206, 237–246. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Ibrahim, S.; Khaleel, S. Performance of vacuum poly (propylene) membrane distillation (VMD) for saline water desalination. Chem. Eng. Process. Process Intensif. 2017, 120, 68–80. [Google Scholar] [CrossRef]
- Shatat, M.; Worall, M.; Riffat, S. Opportunities for Solar Water Desalination Worldwide: Review. Sustain. Cities Soc. 2013, 9, 67–80. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Ibrahim, S.; Hashim, F. Experimental and theoretical investigation of air gap membrane distillation process for water desalination. Chem. Eng. Res. Des. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Jamed, M.; Alanezi, A.A.; Alsalhy, Q.F. Effects of embedding functionalized multi-walled carbon nanotubes and alumina on the direct contact poly (vinylidene fluoride-cohexafluoropropylene) membrane distillation performance. Chem. Eng. Comun. 2019, 206, 1035–1057. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Mjalli, F.S.; Atieh, M.A. PVDF-co-HFP/superhydrophobic acetylene-based nanocarbon hybrid membrane for seawater desalination via DCMD. Chem. Eng. Res. Des. 2018, 138, 248–259. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Das, R.; Mjalli, F.S. Embedded high-hydrophobic CNMs prepared by CVD technique with PVDF-co-HFP membrane for application in water desalination by DCMD. Desalin. Water Treat. 2019, 142, 37–48. [Google Scholar] [CrossRef]
- Yarlagadda, S.; Gude, V.; Camacho, L.; Pinappu, S.; Deng, S. Potable water recovery from As, U, and F contaminated ground waters by direct contact membrane distillation process. J. Hazard. Mater. 2011, 192, 1388–1394. [Google Scholar] [CrossRef]
- Rashid, K.; Rahman, S.; Alsalhy, Q. Optimum Operating Parameters for Hollow Fiber Membranes in Direct Contact Membrane Distillation. Arab. J. Sci. Eng. 2016, 16, 2647–2658. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Al Tahar Bouguecha, S. Application of Response Surface Methodology for Modeling and Optimization of Membrane Distillation Desalination Process. J. Ind. Eng. Chem. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Tain, M.; Xie, Y.; Mei, L.X.; Zhao, B.; He, T.; Liu, J. Effective evaporation of CF4 plasma modified PVDF membranes in direct contact membrane distillation. J. Membr. Sci. 2015, 482, 25–32. [Google Scholar] [CrossRef]
- Cheng, D.; Gong, W.; Li, N. Response surface modeling and optimization of direct contact membrane distillation for water desalination. Desalination 2016, 394, 108–122. [Google Scholar] [CrossRef]
- Tomaszewsk, M. Membrane Distillation-Examples of Applications in Technology and Environmental Protection. Pol. J. Environ. Stud. 2000, 9, 27–36. [Google Scholar]
- Winter, D. Membrane Distillation—A Thermodynamic Technological and Economic Analysis. Ph.D. Thesis, University of Kaiserslautern, Kaiserslautern, Germany, 2014. [Google Scholar]
- Sanmartino, J.; Khayet, M.; García-Payo, M. Desalination by Membrane Distillation; University Complutense of Madrid, Emerging Membrane Technology for Sustainable Water Treatment: Madrid, Spain, 2016; pp. 77–109. [Google Scholar]
- Cath, T.; Adams, V.; Childress, A. Experimental study of desalination using direct contact membrane distillation: A new approach to flux enhancement. J. Membr. Sci. 2004, 228, 5–16. [Google Scholar] [CrossRef]
- My, D. Membrane Distillation Application in Purification and Process Intensification. Master’s Thesis, Asian Institute of Technology, School of Environment, Resources and Development, Bangkok, Thailand, 2015. [Google Scholar]
- Phungsai, P. Development of Thermo Philic Anaerobic Membrane Distillation Bioreactor. Master’s Thesis, Asian Institute of Technology School of Environment, Resources and Development, Bangkok, Thailand, 2013. [Google Scholar]
- El-Bourawi, M.; Ding, Z.; Ma, R.; Khayet, M. A Framework for Better Understanding Membrane Distillation Separation Process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Fard, A.; Rhadfi, T.; Khraisheh, M.; Atieh, M.A.; Khraisheh, M.; Hilal, N. Reducing Flux Decline and Fouling of Direct Contact Membrane Distillation by Utilizing Thermal Brine from MSF Desalination Plant. Desalination 2016, 379, 172–181. [Google Scholar] [CrossRef]
- Lawson, K.; Lioyd, D. Membrane distillation. J. Membr. Sci. 1997, 124, 1–5. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Ibrahim, S.; Alsalhy, Q. Modeling and Simulation for Direct Contact Membrane Distillation in Hollow Fiber Modules. AIChE J. 2013, 59, 589–603. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Gray, S. Effect of applied pressure on performance of PTFE membrane in DCMD. J. Membr. Sci. 2011, 369, 514–525. [Google Scholar] [CrossRef]
- Kullab, A. Desalination Using Membrane Distillation Experimental and Numerical Study. Ph.D. Thesis, Department of Energy Technology, Royal Institute of Technology, Stockholm, Sweden, 2011. [Google Scholar]
- Chen Ching, T.; Ho, C.; Ming Yeh, H. Theoretical Modeling and Experimental Analysis of Direct Contact Membrane Distillation. J. Membr. Sci. 2009, 330, 279–287. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 2. Theoretical analysis, polarization effects and thermal efficiency. J. Membr. Sci. 2013, 433, 180–191. [Google Scholar] [CrossRef]
- Khalifa, A.; Ahmada, H.; Antar, M.; Laoui, T.; Khayet, M. Experimental and theoretical investigations on water desalination using direct contact membrane distillation. Desalination 2017, 404, 22–34. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Effect of pore size distribution and air flux on mass transport in direct contact membrane Distillation. J. Membr. Sci. 2003, 215, 75–85. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Janajreh, I.; Suwwan, D.; Hashaikeh, R. Assessment of Direct Contact Membrane Distillation Under Different Configurations, Velocities and Membrane Properties. Appl. Energy 2016, 185, 1–16. [Google Scholar] [CrossRef]
- Lawal, D.; Khalifa, A. Flux Prediction in Direct Contact Membrane Distillation. Int. J. Mater. Mech. Manuf. 2014, 2, 1–4. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jeong, S.; Lee, S.; Chonc, H.; Seockheon, L. Structural analysis and modeling of the commercial high performance composite flat sheet membranes for membrane distillation application. Desalination 2014, 349, 115–125. [Google Scholar] [CrossRef]
- Benyahia, F. Membrane-Distillation in Desalination; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, LLC: New York, NY, USA, 2019. [Google Scholar]
- Wen Cheng, T.; Jung Han, C.; Jen Hwang, K.; Dong Ho, C.; Cooper, W. Influence of Feed Composition on Distillate Flux and Membrane Fouling in Direct Contact Membrane Distillation. Sep. Sci. Technol. 2010, 45, 967–974. [Google Scholar] [CrossRef]
- Seo, J.; Kim, Y.; Kim, J. Spacer optimization strategy for direct contact membrane distillation: Shapes, configurations, diameters, and numbers of spacer filaments. Desalination 2017, 417, 9–18. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Profio, G.D.; Al-Hinai, H.; Drioli, E. Potential of Membrane Distillation in Seawater Desalination: Thermal Efficiency Sensitivity Study and Cost Estimation. J. Membr. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Optimising thermal efficiency of direct contact membrane distillation by brine recycling for small-scale seawater desalination. Desalination 2015, 374, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Criscuoli, A. Improvement of the Membrane Distillation performance through the integration of different configurations. Chem. Eng. Res. Des. 2016, 111, 316–322. [Google Scholar] [CrossRef]
- Ren, L.-F.; Xia, F.; Shao, J.; Zhang, X.; Li, J. Experimental investigation of the effect of electrospinning parameters on properties of superhydrophobic PDMS/PMMA membrane and its application in membrane distillation. Desalination 2017, 404, 155–166. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Thu, K.; Ghaffour, N.; Choon Ng, K. Performance investigation of a solar assisted direct contact membrane distillation system. J. Membr. Sci. 2013, 427, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Mansouri, J.; Ye, Y.; Chen, V. Effect of templating agents on the properties and membrane distillation performance of TiO2-coated PVDF membranes. J. Membr. Sci. 2014, 450, 48–59. [Google Scholar] [CrossRef]
- Thiel, G.P.; Tow, E.W.; Banchik, L.D.; Chung, H.W.; Lienhard, J.H. Energy consumption in desalinating produced water from shale oil and gas extraction. Desalination 2015, 366, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, M.; Roy, S.; Mitra, S. Flux enhancement in direct contact membrane distillation by implementing carbon nanotube immobilized PTFE membrane. Sep. Purif. Technol. 2016, 161, 136–143. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; AlMarzooqi, F.A.; Arafat, H.A.; Lienhard, V.J.H. Energy efficiency of permeate gap and novel conductive gap membrane distillation. J. Membr. Sci. 2016, 502, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Lin, D.; Ding, C.; Wang, D.; Wang, J. Fabrication and characterization of electrospun superhydrophobic PVDF-HFP/SiNPs hybrid membrane for membrane distillation. Sep. Purif. Technol. 2017, 189, 82–89. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Bouguecha, S.A.T. Direct contact membrane distillation: Capability to desalt raw water. Arab. J. Chem. 2017, 10, S3475–S3481. [Google Scholar] [CrossRef] [Green Version]
- Adnan, S.; Hoang, M.; Wang, H.; Xie, Z. Commercial PTFE membranes for membrane distillation application: effect of microstructure and support material. Desalination 2012, 284, 297–308. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, S.; Zhao, H.; Liu, Y. Distillation membrane constructed by TiO2 nanofiber followed by fluorination for excellent water desalination performance. Desalination 2017, 405, 51–58. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, W.-S.; Choi, J.-S.; Ghaffour, N.; Kim, Y.-D. Dynamic solar-powered multi-stage direct contact membrane distillation system: Concept design, modeling and simulation. Desalination 2018, 435, 278–292. [Google Scholar] [CrossRef]
- Alwatban, A.M.; Alshwairekh, A.M.; Alqsair, U.F.; Alghafis, A.A.; Oztekin, A. Performance improvements by embedded spacer in direct contact membrane distillation–Computational study. Desalination 2019, 470, 114103. [Google Scholar] [CrossRef]
- Floros, I.N.; Kouvelos, E.P.; Pilatos, G.I.; Hadjigeorgiou, E.P.; Gotzias, A.D.; Favvas, E.P.; Sapalidis, A.A. Enhancement of Flux Performance in PTFE Membranes for Direct Contact Membrane Distillation. Polymers 2020, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ren, L.-F.; Shao, J.; Tu, Y.; Ma, Z.; Lin, Y.; He, Y. Fabrication of triple layer composite membrane and its application in membrane distillation (MD): Effect of hydrophobic-hydrophilic membrane structure on MD performance. Sep. Purif. Technol. 2020, 234, 116087. [Google Scholar] [CrossRef]















| Membrane * | Feed Temp. (°C) | Feed Conc. (g/L) | Feed Flow Rate (L/min) | Exp. Flux (kg/m2·h) | Ref. |
|---|---|---|---|---|---|
| PVDF | 50 | 35 | 0.6 | 21 | [32] |
| M4-2(PDMS) | 70 | 35 | 1 | 43 | [44] |
| PTFE | 40–90 | 4.65 | 0.14–100 | 55–72 | [45] |
| PVDF | 80 | 0.45 | 6 | 51.5 | [46] |
| PTFE | 130 | 10 | 0.5 | 195 | [47] |
| PTFE-CNTs | 70 | 34 | - | 69 | [48] |
| PP | 40–60 | - | 0.5–1.7 | 5–25 | [49] |
| PVDF-HFP/SiNPs | 80 | 35 | 1.166 | 48.6 | [50] |
| PP | 85–90 | 10–100 | 25 | 60–79 | [51] |
| PTFE | 60 | Seawater | 4.5 | 45.5 | [52] |
| PTFE + TiO2NF | 50–80 | 0–100 | - | 7–12.2 | [53] |
| PTFE | 38 | Various | 11–22 | 2–5 | [54] |
| PTFE | 70 | 35 | Re = 500–1500 | 47.8–86.8 | [55] |
| PTFE-PP | 60 | 30 | 0.04 | 12.2 | [56] |
| PVDF-PTFE + PET + CS-PEO | 60 | 20 | 0.5 | 19 | [57] |
| PTFE | 45–65 | 0–200 | 0.3–1.07 | Predicted flux 5.1–17.3 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, N.A.M.; Ibrahim, S.S.; Alsalhy, Q.F.; Figoli, A. Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study. Water 2020, 12, 1575. https://doi.org/10.3390/w12061575
Ameen NAM, Ibrahim SS, Alsalhy QF, Figoli A. Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study. Water. 2020; 12(6):1575. https://doi.org/10.3390/w12061575
Chicago/Turabian StyleAmeen, Noor A. Mohammad, Salah S. Ibrahim, Qusay F. Alsalhy, and Alberto Figoli. 2020. "Highly Saline Water Desalination Using Direct Contact Membrane Distillation (DCMD): Experimental and Simulation Study" Water 12, no. 6: 1575. https://doi.org/10.3390/w12061575