Quality of Dissolved Organic Matter Driven by Autotrophic and Heterotrophic Microbial Processes in a Large River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Chlorophyll a Concentration, Bacterial Abundance, and Biomass Production
2.3. Measurement of Dissolved Organic Carbon (DOC) Concentration and Fluorescence
2.4. High-Field FTICR Mass Spectrometry of DOM
2.4.1. Solid Phase Extraction (SPE)
2.4.2. FT-ICR MS Measurement
2.4.3. Data Evaluation
3. Results
3.1. Longitudinal Changes
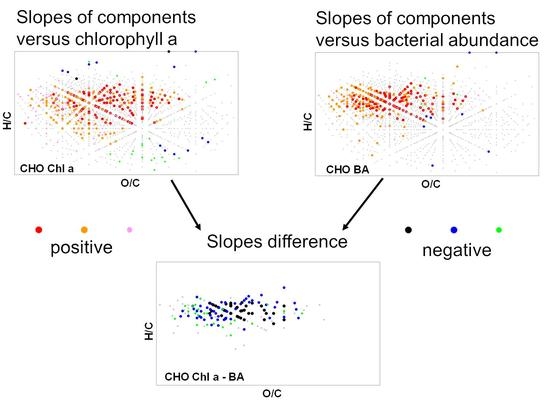
3.2. Relation to Microbial Processes
4. Discussion
4.1. Longitudinal Changes
4.2. Molecular Formula Components and Their Relation to Microbial Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Del Giorgio, P.A.; Pace, M.L. Relative independence of dissolved organic carbon transport and processing in a large temperate river: The Hudson River as both pipe and reactor. Limnol. Oceanogr. 2008, 53, 185–197. [Google Scholar] [CrossRef]
- Wollheim, W.M.; Stewart, R.J.; Aiken, G.R.; Butler, K.D.; Morse, N.B.; Salisbury, J. Removal of terrestrial DOC in aquatic ecosystems of a temperate river network. Geophys. Res. Lett. 2015, 42, 6671–6679. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, R.; Yamashita, Y.; Maie, N.; Cooper, W.T.; Dittmar, T.; Dodds, W.K.; Jones, J.B.; Myoshi, T.; Ortiz-Zayas, J.R.; Podgorski, D.C.; et al. Dissolved organic matter in headwater streams: Compositional variability across climatic regions of North America. Geochim. Cosmochim. Acta 2012, 94, 95–108. [Google Scholar] [CrossRef]
- Parr, T.B.; Cronan, C.S.; Ohno, T.; Findlay, S.E.G.; Smith, S.M.C.; Simon, K.S. Urbanization changes the composition and bioavailability of dissolved organic matter in headwater streams. Limnol. Oceanogr. 2015, 60, 885–900. [Google Scholar] [CrossRef] [Green Version]
- Hosen, J.D.; McDonough, O.T.; Febria, C.M.; Palmer, M.A. Dissolved organic matter quality and bioavailability changes across an urbanization gradient in headwater streams. Environ. Sci. Technol. 2014, 48, 7817–7824. [Google Scholar] [CrossRef]
- Berggren, M.; Del Giorgio, P.A. Distinct patterns of microbial metabolism associated to riverine dissolved organic carbon of different source and quality. J. Geophys. Res. Biogeosci. 2015, 120, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Hale, R.L.; Godsey, S.E. Dynamic stream intermittence explains emergent dissolved organic carbon chemostasis in headwaters. Hydrol. Process. 2019. [Google Scholar] [CrossRef]
- Lynch, L.M.; Sutfin, N.A.; Fegel, S.T.; Boot, C.M.; Covino, T.P.; Wallenstein, M.D. River channel connectivity shifts metabolite composition and dissolved organic matter chemistry. Nat. Comm. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Saiers, J.E.; Sobczak, W.V. Hydrological and biogeochemical controls on watershed dissolved organic matter transport: Pulse-shunt concept. Ecology 2016, 97, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Cory, R.M.; Kaplan, L.A. Biological lability of streamwater fluorescent dissolved organic matter. Limnol. Oceanogr. 2012, 57, 57–1347. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Hertkorn, N.; Frommberger, M.; Witt, M.; Koch, B.P.; Schmitt-Kopplin, P.; Perdue, E.M. Natural organic matter and the event horizon of mass spectrometry. Anal. Chem. 2008, 80, 8908–8919. [Google Scholar] [CrossRef]
- Kim, S.; Kaplan, L.A.; Hatcher, P.G. Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry. Limnol. Oceanogr. 2006, 51, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Stubbins, A.; Spencer, R.G.M.; Chen, H.; Hatcher, P.G.; Mopper, K.; Hernes, P.J.; Mwamba, V.L.; Mangangu, A.M.; Wabakanghanzi, J.N.; Six, J. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 2010, 55, 1467–1477. [Google Scholar] [CrossRef]
- Sleighter, R.L.; Cory, R.M.; Kaplan, L.A.; Abdulla, H.A.N.; Hatcher, P.G. A coupled geochemical and biogeochemical approach to characterize the bioreactivity of dissolved organic matter from a headwater stream. J. Geophys. Res. Biogeosci. 2014, 119, 1520–1537. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Riedel, T.; Niggemann, J.; Vähätalo, A.V.; Dittmar, T.; Jaffe, R. Linking molecular signature of heteroatomic dissolved organic matter to watershed characteristics in world rivers. Environ. Sci. Technol. 2015, 49, 13798–13806. [Google Scholar] [CrossRef]
- Riedel, T.; Zark, M.; Vähätalo, A.V.; Niggemann, J.; Spencer, R.G.M.; Hernes, P.J.; Dittmar, T. Molecular signatures of biogeochemical transformationsin dissolved organic matter from ten world rivers. Front. Earth Sci. 2016, 4, 85. [Google Scholar] [CrossRef] [Green Version]
- Mosher, J.J.; Kaplan, L.A.; Podgorski, D.C.; McKenna, A.M.; Marshall, A.G. Longitudinal shifts in dissolved organic matter chemogeography and chemodiversity within headwater streams: A river continuum reprise. Biogeochemistry 2015, 124, 371–385. [Google Scholar] [CrossRef]
- Kamjunke, N.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Griebler, C.; Brauns, M.; von Tümpling, W.; Weitere, M.; Herzsprung, P. Molecular change of dissolved organic matter and patterns of bacterial activity in a stream along a land-use gradient. Water Res. 2019, 164, 114919. [Google Scholar] [CrossRef] [PubMed]
- Kamjunke, N.; Herzsprung, P.; Neu, T.R. Quality of dissolved organic matter affects planktonic but not biofilm bacterial production in streams. Sci. Total Environ. 2015, 506, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Azam, F. Protein content and protein synthesis rates of planktonic bacteria. Mar. Ecol. Prog. Ser. 1989, 51, 201–213. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Meth. 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Dittmar, T.; Koch, B.; Hertkorn, N.; Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. Meth. 2008, 6, 230–235. [Google Scholar] [CrossRef]
- Raeke, J.; Lechtenfeld, O.J.; Wagner, M.; Herzsprung, P.; Reemtsma, T. Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra. Environ. Sci. Process. Impacts 2016, 18, 918–927. [Google Scholar] [CrossRef]
- Lechtenfeld, O.J.; Kattner, G.; Flerus, R.; McCallister, S.L.; Schmitt-Kopplin, P.; Koch, B.P. Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean. Geochim. Cosmochim. Acta 2014, 126, 321–337. [Google Scholar] [CrossRef] [Green Version]
- Herzsprung, P.; Hertkorn, N.; von Tümpling, W.; Harir, M.; Friese, K.; Schmitt-Kopplin, P. Molecular formula assignment for dissolved organic matter (DOM) using high-field FT-ICR-MS: Chemical perspective and validation of sulphur-rich organic components (CHOS) in pit lake samples. Anal. Bioanal. Chem. 2016, 408, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.P.; Kattner, G.; Passow, U. Molecular insights into the microbial formation of marine dissolved organic matter: Recalcitrant or labile? Biogeosciences 2014, 11, 4173–4190. [Google Scholar] [CrossRef] [Green Version]
- Osterholz, H.; Kirchman, D.L.; Niggemann, J.; Dittmar, T. Environmental drivers of dissolved organic matter molecular composition in the Delaware Estuary. Front. Earth Sci. 2016, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Kamjunke, N.; von Tümpling, W.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Norf, H.; Weitere, M.; Herzsprung, P. A new approach for evaluating transformations of dissolved organic matter (DOM) via high-resolution mass spectrometry and relating it to bacterial activity. Water Res. 2017, 123, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hardenbicker, P.; Weitere, M.; Ritz, S.; Schöll, F.; Fischer, H. Longitudinal plankton dynamics in the rivers Rhine and Elbe. River Res. Appl. 2016, 32, 1264–1278. [Google Scholar] [CrossRef]
- Bowes, M.J.; Gozzard, E.; Johnson, A.C.; Scarlett, P.M.; Roberts, C.; Read, D.S.; Armstrong, L.K.; Harman, S.A.; Wickham, H.D. Spatial and temporal changes in chlorophyll—A concentration in the River Thames basin, UK: Are phosphorus concentrations beginning to limit phytoplankton biomass? Sci. Total Environ. 2012, 426, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Begum, M.S.; Jin, H.; Jang, I.; Lee, J.-M.; Oh, H.B.; Park, J.-H. Optical und molecular-level characterization of fluvial organic matter biodegradation in a highly urbanized river system. Biogeosci. Disc. 2017. [Google Scholar] [CrossRef] [Green Version]
- Begum, M.S.; Jang, I.; Lee, J.-M.; Oh, H.B.; Jin, H.; Park, J.-H. Synergistic effects of urban tributary mixing and dissolved organic matter biodegradation in an impounded river system. Sci. Total Environ. 2019, 676, 105–119. [Google Scholar] [CrossRef]
- Creed, I.F.; McKnight, D.M.; Pellerin, B.A.; Green, M.B.; Bergamaschi, B.A.; Aiken, G.R.; Burns, D.A.; Findlay, S.E.G.; Shanley, J.B.; Striegl, R.G.; et al. The river as a chemostat: Fresh perspectives on dissolved organic matter flowing down the river continuum. Can. J. Fish. Aquat. Sci. 2015, 72, 1272–1285. [Google Scholar] [CrossRef] [Green Version]
- Ward, N.D.; Bianchi, T.S.; Medeiros, P.M.; Seidel, M.; Richey, J.E.; Keil, R.; Sawakuchi, H.O. Where carbon goes when water flows: Carbon cycling across the aquatic continuum. Front. Mar. Sci. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Kujawinski, E.B.; Del Vecchio, R.; Blough, N.V.; Klein, G.C.; Marshall, A.G. Probing molecular-level transformations of dissolved organic matter: Insights on photochemical degradation and protozoan modification of DOM from electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar. Chem. 2004, 92, 23–37. [Google Scholar] [CrossRef]
- Lechtenfeld, O.J.; Hertkorn, N.; Shen, Y.; Witt, M.; Benner, R. Marine sequestration of carbon in bacterial metabolites. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzsprung, P.; Osterloh, K.; von Tümpling, W.; Harir, M.; Hertkorn, N.; Schmitt-Kopplin, P.; Meissner, R.; Bernsdorf, S.; Friese, K. Differences in DOM of rewetted and natural peatlands—results from high-field FT-ICR-MS and bulk optical parameters. Sci. Total Environ. 2017, 586, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Osterholz, H.; Koschinsky, A.; Dittmar, T. Riverine mixing at the molecular scale—An ultrahigh-resolution mass spectrometry study on dissolved organic matter and selected metals in the Amazon confluence zone (Manaus, Brazil). Org. Geochem. 2019, 129, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Gonsior, M.; Zwartjes, M.; Cooper, W.J.; Song, W.; Ishida, K.P.; Tseng, L.Y.; Jeung, M.K.; Rosso, D.; Hertkorn, N.; Schmitt-Kopplin, P. Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry. Water Res. 2011, 45, 2943–2953. [Google Scholar] [CrossRef]
- Lechtenfeld, O.J.; Koch, B.P.; Gasparovic, B.; Frka, S.; Witt, M.; Kattner, G. The influence of salinity on the molecular and optical properties of surface microlayers in a karstic estuary. Mar. Chem. 2013, 150, 25–38. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamjunke, N.; Lechtenfeld, O.J.; Herzsprung, P. Quality of Dissolved Organic Matter Driven by Autotrophic and Heterotrophic Microbial Processes in a Large River. Water 2020, 12, 1577. https://doi.org/10.3390/w12061577
Kamjunke N, Lechtenfeld OJ, Herzsprung P. Quality of Dissolved Organic Matter Driven by Autotrophic and Heterotrophic Microbial Processes in a Large River. Water. 2020; 12(6):1577. https://doi.org/10.3390/w12061577
Chicago/Turabian StyleKamjunke, Norbert, Oliver J. Lechtenfeld, and Peter Herzsprung. 2020. "Quality of Dissolved Organic Matter Driven by Autotrophic and Heterotrophic Microbial Processes in a Large River" Water 12, no. 6: 1577. https://doi.org/10.3390/w12061577