The Chemical State and Occupancy of Radiogenic Pb, and Crystallinity of RW-1 Monazite Revealed by XPS and TEM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Analytical Methods
3. Results
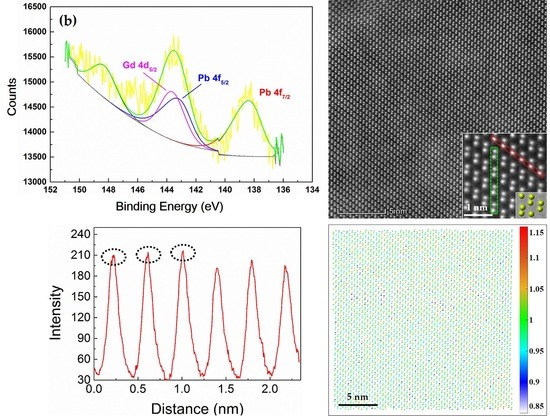
3.1. XPS Results
3.2. Chemical and Mineralogical Features of Monazite-Ce (SEM and RM)
3.3. Atomic Structure of Radiogenic Pb in Monazite (TEM)
4. Discussion
4.1. The Crystallinity of RW-1 Monazite-Ce
4.2. The Chemical State of Radiogenic Pb
4.3. The Nanoscale Distribution of Pb and Th
5. Conclusions
- (1)
- The TEM and STEM-HAADF data demonstrated that the studied natural monazite is well crystalline in most areas. Meanwhile, some distorted lattices induced from self-radiation were also observed, which resulted in a broadening band of RM analysis.
- (2)
- The radiogenic Pb exists as Pb2+ in a current state.
- (3)
- The STEM-HAADF data revealed that Pb atom substitute for the Ce atoms within the monazite crystal lattice. A normalized intensity mapping was developed for the STEM-HAADF images to better visualize the distribution of Pb and Th.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parrish, R.R.; Tirrul, R. U-Pb age of the Baltoro granite, northwest Himalaya, and implications for monazite U-Pb systematics. Geology 1989, 17, 1076–1079. [Google Scholar] [CrossRef]
- Harrison, T.M.; Catlos, E.J.; Montel, J.M. U-Th-Pb Dating of Phosphate Minerals. Rev. Mineral. Geochem. 2002, 48, 524–558. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.L.; Li, X.H.; Lan, Z.W.; Guo, C.L. Monazite and xenotime U–Th–Pb geochronology by ion microprobe dating highly fractionated granites at Xihuashan tungsten mine, SE China. Contrib. Mineral. Petr. 2013, 166, 65–80. [Google Scholar] [CrossRef]
- Meldrum, A.; Boatner, L.A.; Weber, W.J.; Ewing, R.C. Radiation damage in zircon and monazite. Geochim. Cosmochim. Acta 1998, 62, 2509–2520. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Wirth, R.; Deutsch, A.; Schärer, U. Microstructure of 24-1928 Ma concordant monazites; implications for geochronology and nuclear waste deposits. Geochim. Cosmochim. Acta 2004, 68, 2517–2527. [Google Scholar] [CrossRef]
- Vance, E.R.; Zhang, Y.; McLeod, T.; Davis, J. Actinide valences in xenotime and monazite. J. Nucl. Mater. 2011, 409, 221–224. [Google Scholar] [CrossRef]
- Schärer, U.; Xu, R.H.; Allègre, C.J. U-(Th)-Pb systematics and ages of Himalayan leucogranites, South Tibet. Earth. Plan. Sci. Lett. 1986, 77, 35–48. [Google Scholar] [CrossRef]
- Smith, H.A.; Barreiro, B. Monazite U-Pb dating of staurolite grade metamorphism in pelitic schists. Contrib. Mineral. Petr. 1990, 105, 602–615. [Google Scholar] [CrossRef]
- Cocherie, A.; Legendre, O.; Peucat, J.J.; Kouamelan, A.N. Geochronology of polygenetic monazites constrained by in situ electron microprobe Th-U-total lead determination implications for lead behaviour in monazite. Geochim. Cosmochim. Ac. 1998, 62, 2475–2497. [Google Scholar] [CrossRef]
- Bingen, B.; Breemen, O.V. U-Pb monazite ages in amphibolite-to granulite-facies orthogneiss reflect hydrous mineral breakdown reactions: Sveconorwegian Province of SW Norway. Contrib. Mineral. Petr. 1998, 132, 336–353. [Google Scholar] [CrossRef]
- Asami, M.; Suzuki, K.; Grew, E.S. Monazite and Zircon Dating by the Chemical Th-U-Total Pb Isochron Method (CHIME) from Alasheyev Bight to the Sør Rondane Mountains, East Antarctica: A Reconnaissance Study of the Mozambique Suture in East Queen Maud Land. J. Geol. 2005, 113, 59–82. [Google Scholar] [CrossRef]
- Goncalves, P.; Christian, N.; Montel, J.M. Petrology and in situ U-Th-Pb Monazite Geochronology of Ultrahigh-Temperature Metamorphism from the Andriamena Mafic Unit, North-Central Madagascar. Significance of a Petrographical P-T Path in a Polymetamorphic Context. J. Petrol. 2004, 45, 1923–1957. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, D.P.; Bowring, S.A. U-Pb systematics of monazite and xenotime case: Studies from the Paleoproterozoic of the Grand Canyon, Arizona. Contrib. Mineral. Petr. 1997, 127, 87–103. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Goncalves, P.; Wirth, R.; Deutsch, A. Transmission electron microscope study of polyphase and discordant monazites: Site-specific specimen preparation using the focused ion beam technique. Geology 2003, 3, 973–976. [Google Scholar] [CrossRef]
- Fougerouse, D.; Reddy, S.M.; Saxey, D.W.; Erickson, T.M.; Kirkland, C.L.; Rickard, W.D.A.; Seydoux-Guillaume, A.M.; Clark, C.; Buick, I.S. Nanoscale distribution of Pb in monazite revealed by atom probe microscopy. Chem. Geol. 2018, 479, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Seydoux-Guillaume, A.M.; Fougerouse, D.; Laurent, A.T.; Gardés, E.; Reddy, S.M.; Saxey, D.W. Nanoscale resetting of the Th/Pb system in an isotopically-closed monazite grain: A combined atom probe and transmission electron microscopy study. Geosci. Front. 2019, 10, 65–76. [Google Scholar] [CrossRef]
- Catlos, E.J.; Miller, N.R. Ion microprobe 208Th-208Pb ages from high common Pb monazite, Morefield Mine, Amelia County, Virginia: Implications for Alleghanian tectonics. Am. J. Sci. 2016, 316, 470–503. [Google Scholar] [CrossRef]
- Kramers, J.; Frei, R.; Newville, M.; Kober, B.; Villa, I. On the valency state of radiogenic lead in zircon and its consequences. Chem. Geol. 2009, 261, 4–11. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, Y.; Horie, K.; Shimizu, H.; Murakami, T. Determination of the oxidation state of radiogenic Pb in natural zircon using X-ray absorption near-edge structure. Phys. Chem. Miner. 2010, 37, 249–254. [Google Scholar] [CrossRef]
- Dubrail, J.; Farges, F.; Gautron, L.; Harfouche, M.; Borca, C.; Grolimund, D. Pb in naturally irradiated monazites and zircons. J. Phys.: Conf. Ser. 2009, 190. [Google Scholar] [CrossRef] [Green Version]
- Podor, R.; Cuney, M. Experimental study of Th-bearing LaPO4 (780 °C, 200 MPa): Implications for monazite and actinide orthophosphate stability. Am. Mineral. 1997, 82, 765–771. [Google Scholar] [CrossRef]
- Ni, Y.X.; Hughes, J.M.; Mariano, A.N. Crystal chemistry of the monazite and xenotime structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Donovan, J.J.; Hanchar, J.M.; Picolli, P.M.; Schrier, M.D.; Boatner, L.A.; Jarosewich, E. A re-examination of the rare-earth-element orthophosphate standards in use for electron-microprobe analysis. Can. Mineral. 2003, 41, 221–232. [Google Scholar] [CrossRef]
- Taylor, B.E.; Friedrichsen, H. Oxygen and hydrogen isotope disequilibria in the Landsverk I pegmatite, Evje, southem Norway: Evidence for anomalous hydrothermal fluids. Nor. Geogr. Tidsskr. 1983, 63, 199–209. [Google Scholar]
- Ling, X.X.; Huyskens, M.H.; Li, Q.L.; Yin, Q.Z.; Werner, R.; Liu, Y.; Tang, G.Q.; Yang, Y.N.; Li, X.H. Monazite RW-1: A homogenous natural reference material for SIMS U–Pb and Th–Pb isotopic analysis. Miner. Petrol. 2016, 111, 163–172. [Google Scholar] [CrossRef]
- Wu, L.G.; Li, X.H.; Ling, X.X.; Yang, Y.H.; Li, C.F.; Li, Y.L.; Mao, Q.; Li, Q.L.; Putlitz, B. Further Characterization of the RW-1 Monazite: A New Working Reference Material for Oxygen and Neodymium Isotopic Microanalysis. Minerals 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, O.P.; Vasilyev, L.A.; Naumkin, A.V.; Kantsel, V.V. XPS studies of natural monazite and relative compounds under ion bombardment. Appl. Surf. Sci. 1993, 72, 307–312. [Google Scholar] [CrossRef]
- Glorieux, B.; Berjoan, R.; Matecki, M.; Kammouni, A.; Perarnau, D. XPS analyses of lanthanides phosphates. Appl. Surf. Sci. 2007, 253, 3349–3359. [Google Scholar] [CrossRef]
- Apostol, N.G.; Stoflea, L.E.; Lungu, G.A.; Tache, C.A.; Popescu, D.G.; Pintilie, L.; Teodorescu, C.M. Band bending at free Pb(Zr,Ti)O3 surfaces analyzed by X-ray photoelectron spectroscopy. Mater. Sci. Eng. B 2013, 178, 1317–1322. [Google Scholar] [CrossRef]
- Rafiuddin, M.R.; Mueller, E.; Grosvenor, A.P. X-ray Spectroscopic Study of the Electronic Structure of Monazite- and Xenotime-Type Rare-Earth Phosphates. J. Phys. Chem. C 2014, 118, 18000–18009. [Google Scholar] [CrossRef]
- Rondon, S.; Sherwood, P.M.A. Core Level and Valence Band Spectra of PbO by XPS. Surf. Sci. Spectra 1998, 5, 97–103. [Google Scholar] [CrossRef]
- Rondon, S.; Sherwood, P.M.A. Core Level and Valence Band Spectra of Pb3O4 by XPS. Surf. Sci. Spectra 1998, 5, 90–96. [Google Scholar] [CrossRef]
- Rondon, S.; Sherwood, P.M.A. Core Level and Valence Band Spectra of PbO2 by XPS. Surf. Sci. Spectra 1998, 5, 104–110. [Google Scholar] [CrossRef]
- Richter, D.K.; Görgen, P.; Götte, T. Monazite cathodoluminescence—A new tool for heavy mineral analysis of siliciclastic sedimentary rocks. Sediment. Geol. 2008, 209, 36–41. [Google Scholar] [CrossRef]
- Vaggelli, G.; Cossio, R.; Petrelli, M.; Rossetti, P. Combined cathodoluminescence spectroscopy, electron microprobe and laser ablation ICP mass spectrometry analysis: An attempt to correlate luminescence and chemical composition of monazite. Microchim. Acta 2008, 161, 313–321. [Google Scholar] [CrossRef]
- Li, X.H.; Chen, Y.; Tchouankoue, J.P.; Liu, C.Z.; Li, J.; Ling, X.X.; Tang, G.Q.; Liu, Y. Improving geochronological framework of the Pan-African orogeny in Cameroon: New SIMS zircon and monazite U-Pb age constraints. Precambrian Res. 2017, 294, 307–321. [Google Scholar] [CrossRef]
- Ruschel, K.; Nasdala, L.; Kronz, A.; Hanchar, J.M.; Többens, D.M.; Škoda, R.; Finger, F.; Möller, A. A Raman spectroscopic study on the structural disorder of monazite-(Ce). Miner. Petrol. 2012, 105, 41–55. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Wirth, R.; Nasdala, L.; Gottschalk, M.; Montel, J.M.; Heinrich, W. An XRD, TEM and Raman study of experimentally annealed natural monazite. Phys. Chem. Miner. 2002, 29, 240–253. [Google Scholar] [CrossRef]
- Pennycook, S.J.; Jesson, D.E. Atomic resolution Z-contrast imaging of interfaces. Acta metall. Mater. 1992, 40, S149–S159. [Google Scholar] [CrossRef]
- Pennycook, S.J.; Boatner, L.A. Chemically Sensitive Structure-Imaging with a Scanning Transmission Electron Microscope. Nature 1988, 336, 565–567. [Google Scholar] [CrossRef]
- Utsunomiya, S.; Palenik, C.S.; Valley, J.W.; Cavosie, A.J.; Wilde, S.A.; Ewing, R.C. Nanoscale occurrence of Pb in an Archean zircon. Geochim. Cosmochim. Ac. 2004, 68, 4679–4686. [Google Scholar] [CrossRef]
- Weber, W.J. Radiation-induced defects and amorphization in zircon. J. Mater. Res. 1990, 5. [Google Scholar] [CrossRef]
- Murakami, T.; Chakoumakos, B.C.; Ewing, R.C.; Lumpkin, G.R.; Weber, W.J. Alpha-decay event damage in zircon. Am. Mineral. 1991, 76. [Google Scholar] [CrossRef]
- Omel’yanenko, B.I.; Livshits, T.S.; Yudintsev, S.V.; Nikonov, B.S. Natural and artificial minerals as matrices for immobilization of actinides. Geol. Ore Depos. 2007, 49, 173–193. [Google Scholar] [CrossRef]
- Cherniak, D.J.; Watson, E.B.; Grove, M.; Harrison, T.M. Pb diffusion in monazite: A combined RBS/SIMS study. Geochim. Cosmochim. Ac. 2004, 68, 829–840. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar] [CrossRef]
- Catlos, E.J. Versatile Monazite: Resolving geological records and solving challenges in materials science: Generalizations about monazite: Implications for geochronologic studies. Am. Mineral. 2013, 98, 819–832. [Google Scholar] [CrossRef]
- Boatner, L.A.; Sales, B.C. Radioactive Waste Forms for the Future: Monazite; Elsevier Science Publishers: Amsterdam, The Netherlanders, 1988; pp. 495–564. [Google Scholar]
- Meldrum, A.; Boatner, L.A.; Ewing, R.C. Electron-irradiation-induced nucleation and growth in amorphous LaPO4, ScPO4, and zircon. J. Mater. Res. 1997, 12, 1816–1827. [Google Scholar] [CrossRef]
- Deschanels, X.; Seydoux-Guillaume, A.M.; Magnin, V.; Mesbah, A.; Tribet, M.; Moloney, M.P.; Serruys, Y.; Peuget, S. Swelling induced by alpha decay in monazite and zirconolite ceramics: A XRD and TEM comparative study. J. Nucl. Mater. 2014, 448, 184–194. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Deschanels, X.; Baumier, C.; Neumeier, S.; Weber, W.J.; Peuget, S. Why natural monazite never becomes amorphous: Experimental evidence for alpha self-healing. Am. Mineral. 2018, 103, 824–827. [Google Scholar] [CrossRef]
- Nasdala, L.; GrÖtzschel, R.; Probst, S.; Bleisteiner, B. Irradiation damage in monazite-(Ce): An example to establish the limits of Raman confocality and depth resolution. Can. Mineral. 2010, 48, 351–359. [Google Scholar] [CrossRef]
- Black, L.P.; Fitzgerald, J.D.; Harley, S.L. Pb isotopic composition color and microstructure of monazites from a polymetamorphic rock in Antarctica. Contrib. Mineral. Petr. 1984, 85, 141–148. [Google Scholar] [CrossRef]
- Syverson, D.D.; Etschmann, B.; Liu, W.; Ram, R.; Mei, Y.; Lanzirotti, T.; Mercadier, J.; Brugger, J. Oxidation state and coordination environment of Pb in U-bearing minerals. Geochim. Cosmochim. Ac. 2019, 265, 109–131. [Google Scholar] [CrossRef]
- Mi, J.X.; Shen, J.C.; Liang, J.; Pan, B.M. The Crystal Strueture Refinement of Monazite-(Ce) and Xenotime-(Y). Acta Petrol. Mineral. 1996, 15, 53–59. [Google Scholar]
- Boatner, L.A. Synthesis, Structure, and Properties of Monazite, Pretulite, and Xenotime. Rev. Mineral. Geochem. 2002, 48, 87–121. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Study of Inter Atomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.P.; Shen, Z.J.; Li, X.B.; Wang, C.S.; Chen, Y.J.; Li, J.X.; Zhang, J.X.; Zhang, Z.; Zhang, S.B.; et al. Vacancy Structures and Melting Behavior in Rock-Salt GeSbTe. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, W.; Shen, Z.J.; Chen, Y.J.; Li, J.X.; Zhang, S.B.; Zhang, Z.; Wuttig, M.; Mazzarello, R.; Ma, E.; et al. Element-resolved atomic structure imaging of rocksalt Ge2Sb2Te5 phase-change material. Appl. Phys. Lett. 2016, 108, 191902. [Google Scholar] [CrossRef] [Green Version]







| B.E. (eV) 1 | Chip1 | Chip2 | PbO | Pb3O4 | PbO2 |
|---|---|---|---|---|---|
| Pb 4f7/2 | 138.16 | 138.04 | 138.0 | 137.4 | 137.3 |
| Pb 4f5/2 | 143.02 | 142.9 | 142.9 | 142.2 | 142.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Li, Q.-L.; Zhang, B.; Wang, P.; Gu, L.-X.; Ling, X.-X.; Fei, C.-H.; Li, J.-H. The Chemical State and Occupancy of Radiogenic Pb, and Crystallinity of RW-1 Monazite Revealed by XPS and TEM. Minerals 2020, 10, 504. https://doi.org/10.3390/min10060504
Tang X, Li Q-L, Zhang B, Wang P, Gu L-X, Ling X-X, Fei C-H, Li J-H. The Chemical State and Occupancy of Radiogenic Pb, and Crystallinity of RW-1 Monazite Revealed by XPS and TEM. Minerals. 2020; 10(6):504. https://doi.org/10.3390/min10060504
Chicago/Turabian StyleTang, Xu, Qiu-Li Li, Bin Zhang, Peng Wang, Li-Xin Gu, Xiao-Xiao Ling, Chen-Hui Fei, and Jin-Hua Li. 2020. "The Chemical State and Occupancy of Radiogenic Pb, and Crystallinity of RW-1 Monazite Revealed by XPS and TEM" Minerals 10, no. 6: 504. https://doi.org/10.3390/min10060504