Abstract
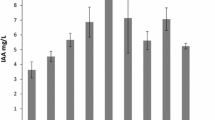
Plant growth promoting rhizobacteria (PGPR) are important for agriculture through their activity in stimulating and facilitating plant growth. The rhizobacteria were screened for molecular characterization and followed by their indole acetic acid (IAA) production, phosphate solubility, antibiosis activity. In this study, 162 soil samples were collected from the cocoa rhizosphere to isolate Bacillus subtilis strains using Mössel agar medium with an additional egg yolk and identified by sequencing the ytcP gene. The ability of each strain to form biofilms was obtained in a tube. Indole-3-acetic acid (IAA) production was estimated in Yeast Peptone Dextrose (YPB) broth. Phosphates were solubilized by each strain on Pikovskaya agar medium. The detection of lipopeptide genes using the molecular method has established the possession of isolates by antimicrobial genes. Fifty (50) B. subtilis strains were isolated and identified using the ytcP gene. Ninety percent (90%) of the strains were able to form a biofilm. All isolates produced an IAA. Forty (40 (80%)) of B. subtilis were solubilized phosphate with phosphate solubilizing index (PSI) of 0 to 97.33 ± 0.70%. Of all B. subtilis strains, 45 (90%) have the srfAA gene, 19 (38%) have the fenD gene and 12 (24%) have the ituC gene. B. subtilis strains from cocoa rhizosphere would be beneficial for agricultural production by their PGPR activities.








Similar content being viewed by others
References
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–5119
Ongena M (2014) Biopesticides : une protection plus naturelle pour les cultures. Université de Liège - https://reflexions.ulg.ac.be. p 10
Khan MS, Zaidi A, Wani PA, Oves M (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:1–19
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi - current perspective. Arch Agron Soil Sci 56:73–98
Gupta G, Shailendra SP, Narendra KA, Sunil KS, Vinod S (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microbiol Biochem Techno 1:7
Lynch JM (1985) Origin, nature and biological activity of aliphatic substances and growth hormones found in soil. In: Vaughan D, Malcom RE (eds) Soil organic matter and biological activity. Martinus Nijhoff /Dr. W. Junk Publishers, Dordrecht, pp 151–174
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Hindawi Publishing Corporation, London, p 15
Sucuia OA, Florica C, Cornea CP (2015) Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection. Rom Biotechnol Lett 20(5):10737–10750
Huang X, Suo J, Cui Y (2011) Optimization of antimicrobial activity of surfactin and polylysine against Salmonella enteritidis in milk evaluated by a response surface methodology. Foodborne Pathog Dis 8(3):439–443
Płaza GA, Turek A, Król E, Szczygłowska R (2013) Antifungal and antibacterial properties of surfactin isolated from Bacillus subtilis growing on molasses. Afr J Microbiol Res 7(25):3165–3170
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny JL, Thonat P (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systémique resistance in plants. Environ Microbiol 9:1084–1090
Saha D, Purkayastha GD, Ghosh A, Isha M (2012) Isolation and characterization of two new bacillus subtilis strains from the rhizosphere of eggplant as potential biocontrol agents. J Plant Pathol 94(1):109–118
Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting Rhizobacteria. J Microbiol Antimicrob 3(2):34–40
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Srilatha P, Venkateswarlu K (2009) Potential of Rhizosphere microorganisms in phosphate solubilisation. Ecol Environ Conserv 15(1):115–119
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499
Mona AE, Eman FA, Wafaa AH, Nahla MM, Waled ME, Mounir ME (2012) Antiviral Levans from Bacillus spp. Isolated from Hone. InTech 7:195–214
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Mora I, Cabrefiga J, Montesinos E (2011) Antimicrobial peptide genes in Bacillus strains from plant environments. Int Microbiol 14:213–223
Kakar KK, Yongping D, Zarqa N, Guochang S, Abdlwareth A, Hassan M, Shakh A, Li B, Xie G (2014) A novel rhizobacterium Bk7 for biological control of brown sheath rot of rice caused by Pseudomonas fuscovaginae and its mode of action. Eur J Plant Pathol 138:819–834
Christensen GD, Simpson WA, Bisno AL, Beachy EH (1982) Adherence of biofilm producing strains of Staphylococci epidermidis to smooth surfaces. Infect Immun 37:318–326
Ehmann A (1977) The Van Urk-Salkowski reagent-a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr 132:267–276
Adharsini SS, Balaji S, Vivek M (2016) Production, purification and application of plant growth hormones (IAA and gibberellic acid) by Bacillus subtilis. J Biospectra 1(5):1–4
Pikovskaya RI (1948) Mobilization of phosphorus and soil in connection with the vital activity of some microbials pecies. Mikrobiologii 17:362–370
Acknowledgements
The authors would like to thank the Pasteur Institute Abidjan, Cote d’Ivoire for his logistic assistance.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koua, S.H., N’golo, D.C., Alloue-Boraud, W.M. et al. Bacillus subtilis Strains Isolated from Cocoa Trees (Theobroma cacao L.) Rhizosphere for their use as Potential Plant Growth Promoting Rhizobacteria in Côte d’Ivoire. Curr Microbiol 77, 2258–2264 (2020). https://doi.org/10.1007/s00284-020-02027-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02027-x