Abstract
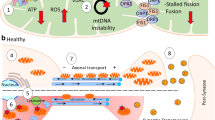
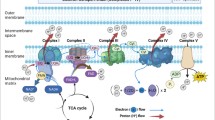
Mitochondrial dysfunction plays a very vital role in the pathogenesis of Alzheimer’s disease (AD). Several shreds of evidence have indicated that the mitochondrial function is severely compromised under AD pathogenesis. Most of the recent therapeutic strategies have been conversed to treat AD by pinpointing the pathways involved in the pathophysiology of AD. In AD, mitochondria progressively lose their proper functions that are ultimately responsible for their accumulation and removal via the autophagic process, which is called mitophagy that further worsens the progression of this incapacitating disease. Preclinical and clinical studies have suggested that mitochondrial dysfunction along with mitophagy significantly contributes to the accumulation of amyloid-beta (Aβ) fibrils and hyperphosphorylated tau protein tangles which lead to synaptic dysfunctions and cognitive impairments such as memory loss through reactive oxygen species (ROS)–mediated pathway. The present review is intended to discuss the recent advancements in the frontiers of mitochondrial dysfunction and consequent therapeutic strategies that have been employed to treat AD.



Similar content being viewed by others
References
Santos R, Correia S, Wang X, Perry G, Smith M, Moreira P, Zhu XA (2010) Synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J Alzheimers Dis 20(2):401–412. https://doi.org/10.3233/JAD-2010-100666
Association, A.S (2017) Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 13(4):325–373. https://doi.org/10.1016/j.jalz.2017.02.001
Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurology 71(4):505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Kumar, A. and Singh, A., Ekavali. (2015). A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacological. Reports. 67(2), 195–203. doi: https://doi.org/10.1016/j.pharep.2014.09.004.
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 20(s2):S265–S279. https://doi.org/10.3233/JAD-2010-100339
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta 1842(8):1219–1231. https://doi.org/10.1016/j.bbadis.2013.09.010
Yellen (2018) Fueling thought: management of glycolysis and oxidative phosphorylation inneuronal metabolism. J Cell Biol 217(7):2235–2246
Hoye AT et al (2008) Targeting mitochondria. Acc. Chem. Res 41(1):87–97. https://doi.org/10.1021/ar700135m
Tan BL, Norhaizan ME, Winnie-Pui-Pui Liew HS (2018) Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Frontiers in Pharmacology 9. https://doi.org/10.3389/fphar.2018.01162
Ferreira IL et al (2010) Multiple defects in energy metabolism in Alzheimer’s disease. Current Drug Targets 11(10):1193–1206. https://doi.org/10.2174/1389450111007011193
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658. https://doi.org/10.1111/j.1471-4159.2006.03907.x
Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. https://doi.org/10.1016/j.biopha.2015.07.025
Mosconi L (2013) Glucose metabolism in normal aging and Alzheimer’s disease: methodological and physiological considerations for PET studies. Clinical and translational imaging 1(4):217–233. https://doi.org/10.1007/s40336-013-0026-y
Slow EJ, Graham RK, Hayden MR (2006) To be or not to be toxic: aggregations in Huntington and Alzheimer disease. Trends Genet 22:408–411. https://doi.org/10.1016/j.tig.2006.05.008
Smith MA (1998) Alzheimer disease. Int Rev Neurobiol 42:1–54
Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V et al (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156:15–20
Kopsidas G, Kovalenko SA, Heffernan DR et al (2000) Tissue mitochondrial DNA changes. A stochastic system. Ann NY Acad Sci 908:226–243. https://doi.org/10.1111/j.1749-6632.2000.tb06650.x
Bergamini E, Cavallini G, Donati A, Gori Z (2004) The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol 36:2392–2404. https://doi.org/10.1016/j.biocel.2004.05.007
Davis AF, Clayton DA (1996) In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol 135:883–893. https://doi.org/10.1083/jcb.135.4.883
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45(7–8):466–472. https://doi.org/10.1016/j.exger.2010.01.003
Kao LP, Wolvetang EJ (2017) Mitochondrial dysfunction and mitophagy in neurodegenerative diseases. Cell Dev Biol 6(184):2. https://doi.org/10.4172/2168-9296.1000184
Lezi E, Swerdlow RH (2012) Mitochondria in neurodegeneration. Adv Exp Med Biol 942:269–286. https://doi.org/10.1007/978-94-007-2869-1_12
Casley C et al (2001) β-Amyloid inhibits integrated mitochondrial respiration and key enzyme activities. Journal of Neurochemistry 80(1):91–100. https://doi.org/10.1046/j.0022-3042.2001.00681.x
Zhao, Y. and Zhao, B. (2013). Oxidative stress and the pathogenesis of Alzheimer’s disease Oxidative medicine and cellular longevity 2013, 316523. doi: https://doi.org/10.1155/2013/316523.
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342(3):619–630. https://doi.org/10.1124/jpet.112.192138
Pagani, L. and Eckert, A. (2011). Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis 2011, 925050. doi: https://doi.org/10.4061/2011/925050, 12.
Shi Q, Gibson GE (2007) Oxidative stress and transcriptional regulation in Alzheimer’s disease. Alzheimer Dis Assoc Disord 21(4):276–291. https://doi.org/10.1097/WAD.0b013e31815721c3
Reddy PH (2011) Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res 1415:136–148. https://doi.org/10.1016/j.brainres.2011.07.052
Baloh RH (2008) Mitochondrial dynamics and peripheral neuropathy. Neuroscientist 14(1):12–18. https://doi.org/10.1177/1073858407307354
Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H et al (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60(8):759–767. https://doi.org/10.1093/jnen/60.8.759
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19(8):823–835. https://doi.org/10.1089/ars.2012.5027
Platt TL, Reeves VL, Murphy MP (2013) Transgenic models of Alzheimer’s disease: better utilization of existing models through viral transgenesis. Biochim Biophys Acta 1832(9):1437–1448. https://doi.org/10.1016/j.bbadis.2013.04.017
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X et al (2004) ABAD directly links Aß to mitochondrial toxicity in Alzheimer’s disease. Science. 304(5669):448–452. https://doi.org/10.1126/science.1091230
Yao J, Chen S, Mao Z, Cadenas E, Brinton RD (2011a) 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PLoS One 6(7):e21788. https://doi.org/10.1371/journal.pone.0021788
Yao J, du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D et al (2011b) Inhibition of amyloid-β (Aβ) peptide-binding alcohol dehydrogenase-Aβ interaction reduces Aβ accumulation and improves mitochondrial function in a mouse model of Alzheimer’s Disease. J Neurosci 31(6):2313–2320
Wang J-Z, Liu F (2008) Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol 85(2):148–175. https://doi.org/10.1016/j.pneurobio.2008.03.002
Martin L, Latypova X, Terro F (2011) Post-translational modifications of tau protein: implications for Alzheimer’s disease. Neurochem Int 58(4):458–471. https://doi.org/10.1016/j.neuint.2010.12.023
Zempel H et al (2010) Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. Journal of Neuroscience 30(36):11938–11950. https://doi.org/10.1523/JNEUROSCI.2357-10.2010
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166. https://doi.org/10.1016/j.tins.2017.01.002
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121. https://doi.org/10.3233/JAD-161088
Jayapalan S, Natarajan J (2013) The role of CDK5 and GSK3B kinases in hyperphosphorylation of microtubule associated protein tau (MAPT) in Alzheimer’s disease. Bioinformation 9(20):1023–1030. https://doi.org/10.6026/97320630091023
Rogers SL, Gelfand VI (2000) Membrane trafficking, organelle transport, and the cytoskeleton. Current Opinion in Cell Biology 12(1):57–62. https://doi.org/10.1016/S0955-0674(99)00057-5
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103. https://doi.org/10.1523/JNEUROSCI.1357-09.2009
Kandimalla R, Reddy PH (2016) Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim Biophys Acta 1862(4):814–828. https://doi.org/10.1016/j.bbadis.2015.12.018
Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D (2004) The biology of mitochondrial uncoupling proteins. Diabetes. 53(1):S130–S135. https://doi.org/10.2337/diabetes.53.2007.s130
Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 53(1):S110–S118. https://doi.org/10.2337/diabetes.53.2007.s110
Kukat A, Dogan SA, Edgar D, Mourier A, Jacoby C, Maiti P, Mauer J, Becker C et al (2014) Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. PLoS Genet 10(6):e1004385. https://doi.org/10.1371/journal.pgen.1004385
Sreedhar A, Zhao Y (2017) Uncoupling protein 2 and metabolic diseases. Mitochondrion. 34:135–140. https://doi.org/10.1016/j.mito.2017.03.005
Kim I, Lemasters JJ (2010) Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Phys Cell Phys 300(2):C308–C317. https://doi.org/10.1152/ajpcell.00056.2010
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8(1):3–5. https://doi.org/10.1089/rej.2005.8.3
Bolisetty S, Jaimes E (2013) Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 14(3):6306–6344. https://doi.org/10.3390/ijms14036306
Taylor R, Goldman SJ (2011) Mitophagy and disease: new avenues for pharmacological intervention. Curr Pharm Des 17(20):2056–2073
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462(2):245–253. https://doi.org/10.1016/j.abb.2007.03.034
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8(21):2003–2014. https://doi.org/10.3969/j.issn.1673-5374.2013.21.009
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950. https://doi.org/10.1152/physrev.00026.2013
Calabrese V, Cornelius C, Stella AMG, Calabrese EJ (2010) Cellular stress responses, mitostress and carnitine insufficiencies as critical determinants in aging and neurodegenerative disorders: role of hormesis and vitagenes. Neurochem Res 35(12):1880–1915. https://doi.org/10.1007/s11064-010-0307-z
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. https://doi.org/10.1016/j.pneurobio.2013.10.004
Zhang T, Xue L, Li L, Tang C, Wan Z, Wang R, Tan J, Tan Y et al (2016) BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J Biol Chem 291(41):21616–21629. https://doi.org/10.1074/jbc.M116.733410
Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ (2013) The PINK1–Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci 110(16):6400–6405. https://doi.org/10.1073/pnas.1221132110
Sekine S, Kanamaru Y, Koike M, Nishihara A, Okada M, Kinoshita H, Kamiyama M, Maruyama J et al (2012) Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J Biol Chem 287(41):34635–34645. https://doi.org/10.1074/jbc.M112.357509
Scarffe LA, Stevens DA, Dawson VL, Dawson TM (2014) Parkin and PINK1: much more than mitophagy. Trends Neurosci 37(6):315–324. https://doi.org/10.1016/j.tins.2014.03.004
Heo J-M, Ordureau A, Paulo JA, Rinehart J, Harper JW (2015) The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell 60(1):7–20. https://doi.org/10.1016/j.molcel.2015.08.016
Ashrafi G, Schwarz T (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. https://doi.org/10.1038/cdd.2012.81
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580. https://doi.org/10.1038/cdd.2010.191
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. https://doi.org/10.1016/j.molcel.2010.09.023
Karabiyik C, Lee MJ, Rubinsztein DC (2017) Autophagy impairment in Parkinson’s disease. Essays Biochem 61(6):711–720
Guo F, Liu X, Cai H, Le W (2018) Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol 28(1):3–13
Giordano S, Darley-Usmar V, Zhang J (2014) Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol 2:82–90. https://doi.org/10.1016/j.redox.2013.12.013
Shaid S, Brandts CH, Serve H, Dikic I (2013) Ubiquitination and selective autophagy. Cell Death Differ 20(1):21–30. https://doi.org/10.1038/cdd.2012.72
Lionaki E, Markaki M, Palikaras K, Tavernarakis N (2015) Mitochondria, autophagy and age-associated neurodegenerative diseases: new insights into a complex interplay. Biochim Biophys Acta 1847(11):1412–1423. https://doi.org/10.1016/j.bbabio.2015.04.010
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Marchetto MC et al (2011) Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Human Molecular Genetics 20(R2):R109–R115. https://doi.org/10.1093/hmg/ddr336
Moran N (2013) Banking iPS cells. Nature Biotechnology 31:11. https://doi.org/10.1038/nbt0113-11
Jucker M (2010) The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 16(11):1210–1214. https://doi.org/10.1038/nm.2224
Reddy PH, Beal MF (2005) Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev 49:618–632. https://doi.org/10.1016/j.brainresrev.2005.03.004
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010a) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta 1802:2–10. https://doi.org/10.1016/j.bbadis.2009.10.006
Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA (2010b) Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta 1802(1):212–220. https://doi.org/10.1016/j.bbadis.2009.10.007
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/BJ20081386
Ding, W.X., Yin, X.M. (2012). Mitophagy: mechanisms, pathophysiological roles, and analysis Biol. Chem. 393, 547–564. doi: https://doi.org/10.1515/hsz-2012-0119.
Mayer G, Nitsch R, Hoyer S (1990) Effects of changes in peripheral and cerebral glucose metabolism on locomotor activity, learning and memory in adult male rats. Brain Research 532(1-2):95–100. https://doi.org/10.1016/0006-8993(90)91747-5
Salkovic-Petrisic M, Knezovic A, Hoyer S, Riederer P (2013) What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J Neural Transm 120(1):233–252. https://doi.org/10.1007/s00702-012-0877-9
Salkovic-Petrisic M et al (2011) Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm 118(5):765–772. https://doi.org/10.1007/s00702-011-0651-4
Stefanova N, Kozhevnikova O, Vitovtov A, Maksimova K, Logvinov S, Rudnitskaya E, Korbolina E, Muraleva N et al (2014a) Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease. Cell Cycle 13(6):898–909. https://doi.org/10.4161/cc.28255
Stefanova NA et al (2014b) Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease. Cell Cycle 13(6):898–909. https://doi.org/10.4161/cc.28255
Stefanova NA et al (2015) Amyloid accumulation is a late event in sporadic Alzheimer’s disease-like pathology in nontransgenic rats. Oncotarget 6(3):1396. https://doi.org/10.18632/oncotarget.2751
Cheng XR, Zhou WX, Zhang YX (2014) The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res Rev 13:13–37. https://doi.org/10.1016/j.arr.2013.10.002
D’Souza Y et al (2015) Characterization of Aldh2-/-mice as an age-related model of cognitive impairment and Alzheimer’s disease. Molecular Brain 8(1):27. https://doi.org/10.1186/s13041-015-0117-y
Palikaras K, Lionaki E, Tavernarakis N (2015) Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 521:525–528. https://doi.org/10.1038/nature14300
Cai Q, Tammineni P (2016) Alterations in mitochondrial quality control in Alzheimer’s disease. Frontiers in Cellular Neuroscience 10(24). https://doi.org/10.3389/fncel.2016.00024
Uttara B, Singh A, Zamboni P, Mahajan R (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. https://doi.org/10.2174/157015909787602823
Valero-Grinan, T.M. (2014). Mitochondrial biogenesis: pharmacological approaches, https://doi.org/10.2174/138161282035140911142118
Enriquez JA, Lenaz G (2014) Coenzyme q and the respiratory chain: coenzyme q pool and mitochondrial supercomplexes. Molecular syndromology 5(3–4):119–140. https://doi.org/10.1159/000363364
Hernández-Camacho JD et al (2018) Coenzyme Q10 supplementation in aging and disease. Frontiers in Physiology 9:44. https://doi.org/10.3389/fphys.2018.00044
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer’s disease: clinical trials and drug development. The Lancet Neurology 9(7):702–716. https://doi.org/10.1016/S1474-4422(10)70119-8
Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40(5):1305–1313. https://doi.org/10.1007/s00726-011-0851-0
Hawking, Z.L. (2016). Alzheimer’s disease: the role of mitochondrial dysfunction and potential new therapies.Bioscience Horizons: The International Journal of Student Research. 9. https://doi.org/10.1093/biohorizons/hzw014
Kurutas EB (2015) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):71. https://doi.org/10.1186/s12937-016-0186-5
Yi X, Maeda N (2005) Endogenous production of lipoic acid is essential for mouse development. Mol Cell Biol 25(18):8387–8392. https://doi.org/10.1128/MCB.25.18.8387-8392.2005
Van Bulck M et al (2019) Novel approaches for the treatment of Alzheimer’s and Parkinson’s disease. International Journal of Molecular Sciences 20(3):719. https://doi.org/10.3390/ijms20030719
Kuruva CS, Manczak M, Yin X, Ogunmokun G, Reddy AP, Reddy PH (2017) Aqua-soluble DDQ reduces the levels of Drp1 and A β and inhibits abnormal interactions between A β and Drp1 and protects Alzheimer’s disease neurons from A β-and Drp1-induced mitochondrial and synaptic toxicities. Hum Mol Genet 26(17):3375–3395. https://doi.org/10.1093/hmg/ddx226
Reddy, P.H., et al. (2019). Current status of healthy aging and dementia research: a symposium summary. Journal of Alzheimer’s Disease. 1-25. doi: https://doi.org/10.3233/JAD-190252.
Cid-Castro C, Hernandez-Espinosa DR, Moran J (2018) ROS as regulators of mitochondrial dynamics in neurons. Cell Mol Neurobiol 38(5):995–1007. https://doi.org/10.1007/s10571-018-0584-7
Du H, Guo L, Yan SS (2012) Synaptic mitochondrial pathology in Alzheimer’s disease. Antioxid Redox Signal 16(12):1467–1475. https://doi.org/10.1089/ars.2011.4277
Joshi AU et al (2018) Drp1/Fis1 interaction mediates mitochondrial dysfunction, bioenergetic failure and cognitive decline in Alzheimer’s disease. Oncotarget 9(5):6128
Du H et al (2010) Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci 107(43):18670–18675. https://doi.org/10.1073/pnas.1006586107
Raefsky SM, Mattson MP (2017) Adaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistance. Free Radic Biol Med 102:203–216. https://doi.org/10.1016/j.freeradbiomed.2016.11.045
Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB (2010) Short-term fasting induces profound neuronal autophagy. Autophagy. 6(6):702–710. https://doi.org/10.4161/auto.6.6.12376
Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R et al (2016) Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab 23(1):128–142. https://doi.org/10.1016/j.cmet.2015.10.013
Rodger CE, McWilliams TG, Ganley IG (2018) Mammalian mitophagy–from in vitro molecules to in vivo models. FEBS J 285(7):1185–1202. https://doi.org/10.1111/febs.14336
Ryu D et al (2016) Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature Medicine 22(8):879. https://doi.org/10.1038/nm.4132
Cuyàs E et al (2018) Metformin is a direct SIRT1-activating compound: computational modeling and experimental validation. Frontiers in Endocrinology 9:657. https://doi.org/10.3389/fendo.2018.00657
Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA (2015) Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol 15(1):19. https://doi.org/10.1186/s12883-015-0272-x
Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A (2014) Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ (1–42)-induced rat model of Alzheimer’s disease. Free Radic Res 48(2):146–158. https://doi.org/10.3109/10715762.2013.857018
Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H et al (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6):838–847. https://doi.org/10.1016/j.cmet.2012.04.022
Geisler JG, Marosi K, Halpern J, Mattson MP (2017) DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement 13(5):582–591. https://doi.org/10.1016/j.jalz.2016.08.001
Lee HK, Kwon B, Lemere CA, de la Monte S, Itamura K, Ha AY, Querfurth HW (2017) mTORC2 (Rictor) in Alzheimer’s disease and reversal of amyloid-β expression-induced insulin resistance and toxicity in rat primary cortical neurons. J Alzheimers Dis 56(3):1015–1036. https://doi.org/10.3233/JAD-161029
Hensley K, Kursula P (2016) Collapsin response mediator protein-2 (CRMP2) is a plausible etiological factor and potential therapeutic target in Alzheimer’s disease: comparison and contrast with microtubule-associated protein tau. J Alzheimers Dis 53(1):1–14. https://doi.org/10.3233/JAD-160076
Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 376(6540):509–514. https://doi.org/10.1038/376509a0
Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K (2005) CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol 25(22):9920–9935. https://doi.org/10.1128/MCB.25.22.9920-9935.2005
Harris-White ME, Ferbas KG, Johnson MF, Eslami P, Poteshkina A, Venkova K, Christov A, Hensley K (2015) A cell-penetrating ester of the neural metabolite lanthionine ketimine stimulates autophagy through the mTORC1 pathway: evidence for a mechanism of action with pharmacological implications for neurodegenerative pathologies. Neurobiol Dis 84:60–68. https://doi.org/10.1016/j.nbd.2015.03.007
Caccamo A, de Pinto V, Messina A, Branca C, Oddo S (2014) Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 34(23):7988–7998. https://doi.org/10.1523/JNEUROSCI.0777-14.2014
Chu CC, Wang JJ, Chen KT, Shieh JP, Wang LK, Shui HA, Ho ST (2010) Neurotrophic effects of tianeptine on hippocampal neurons: a proteomic approach. J Proteome Res 9(2):936–944. https://doi.org/10.1021/pr900799b
Kodama Y et al (2004) Induction of CRMP-2 by GDNF and analysis of the CRMP-2 promoter region. Biochemical and Biophysical Research Communications 320(1):108–115
Afghah Z, Chen X, Geiger JD (2020) Role of endolysosomes and inter-organellar signaling in brain disease. Neurobiology of Disease 134:104670
Voss et al (2020) AMP-activated protein kinase (AMPK) regulates astrocyte oxidative metabolism by balancing TCA cycle dynamics. Glia. https://doi.org/10.1002/glia.23808
Jones SV, Kounatidis I (2017) Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol 8:1805. https://doi.org/10.3389/fimmu.2017.01805
Rai SN, Birla H, Singh SS, Zahra W, Patil RR, Jadhav JP, Gedda MR, Singh SP (2017a) Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-κB/p-Akt1 signaling pathways. Front Aging Neurosci 9. https://doi.org/10.3389/fnagi.2017.00421
Rai SN, Birla H, Zahra W, Singh SN, Singh SP (2017b) Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp). J Chem Neuroanat 85:27–35. https://doi.org/10.1016/j.jchemneu.2017.06.005
Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, Singh BK, Singh SP (2019) The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res 35:775–795. https://doi.org/10.1007/s12640-019-0003-y
Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E et al (2013) Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13(6):691–705. https://doi.org/10.1016/j.stem.2013.11.006
Mattson MP (2015) Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev 20:37–45. https://doi.org/10.1016/j.arr.2014.12.011
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19(2):181–192. https://doi.org/10.1016/j.cmet.2013.12.008
Sun N, Yun J, Liu J, Malide D, Liu C, Rovira II, Holmström KM, Fergusson MM et al (2015) Measuring in vivo mitophagy. Mol Cell 60(4):685–696. https://doi.org/10.1016/j.molcel.2015.10.009
Julien C, Tremblay C, Émond V, Lebbadi M, Salem N Jr, Bennett DA, Calon F (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 68(1):48–58. https://doi.org/10.1097/NEN.0b013e3181922348
Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP (2013) Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 34(6):1564–1580. https://doi.org/10.1016/j.neurobiolaging.2012.11.020
Geisler JG, et al. DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement 2016.
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R et al (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5(4):e9979. https://doi.org/10.1371/journal.pone.0009979
Alzheimer’s disease. Journal of Neuroscience. 31(6), 2313–2320. doi: https://doi.org/10.1523/JNEUROSCI.4717-10.2011
Fernández PL, Britton GB, Rao KS (2013) Potential immunotargets for Alzheimer’s disease treatment strategies. J Alzheimers Dis 33(2):297–312. https://doi.org/10.3233/JAD-2012-121222
Thellung S et al (2019) Autophagy activator drugs: a new opportunity in neuroprotection from misfolded protein toxicity. International Journal of Molecular Sciences 20(4):–901. https://doi.org/10.3390/ijms20040901
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (1842) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842(8):1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Yang P, Sheng D, Guo Q, Wang P, Xu S, Qian K, Li Y, Cheng Y et al (2020) Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer’s disease. Biomaterials. 238:119844
Acknowledgments
Authors would like to acknowledge UGC Dr. D.S. Kothari Postdoctoral scheme for awarding the fellowship to S.N.R (Ref. No-F.4-2/2006 (BSR)/BL/19-20/0032). The authors also would like to acknowledge Bio-render (www.biorender.com) for providing an efficient platform to create all the figures.
Author information
Authors and Affiliations
Contributions
S.N.R. and B.K.S. designed the study and wrote the manuscript along with creating all the figures. C.S., A.S., and M.P.S. edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rai, S.N., Singh, C., Singh, A. et al. Mitochondrial Dysfunction: a Potential Therapeutic Target to Treat Alzheimer’s Disease. Mol Neurobiol 57, 3075–3088 (2020). https://doi.org/10.1007/s12035-020-01945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01945-y