Abstract
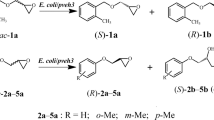
In order to provide more alternative epoxide hydrolases for industrial production, a novel cDNA gene Rpeh-encoding epoxide hydrolase (RpEH) of Rhodotorula paludigena JNU001 identified by 26S rDNA sequence analysis was amplified by RT-PCR. The open-reading frame (ORF) of Rpeh was 1236 bp encoding RpEH of 411 amino acids and was heterologously expressed in Escherichia coli BL21(DE3). The substrate spectrum of expressed RpEH showed that the transformant E. coli/Rpeh had excellent enantioselectivity to 2a, 3a, and 5a–10a, among which E. coli/Rpeh had the highest activity (2473 U/g wet cells) and wonderful enantioselectivity (E = 101) for 8a, and its regioselectivity coefficients, αR and βS, toward (R)- and (S)-8a were 99.7 and 83.2%, respectively. Using only 10 mg wet cells/mL of E. coli/Rpeh, the near-perfect kinetic resolution of rac-8a at a high concentration (1000 mM) was achieved within 2.5 h, giving (R)-8a with more than 99% enantiomeric excess (ees) and 46.7% yield and producing (S)-8b with 93.2% eep and 51.4% yield with high space-time yield (STY) for (R)-8a and (S)-8b were 30.6 and 37.3 g/L/h.







Similar content being viewed by others
References
Archelas A, Iacazio G, Kotik M (2016) Epoxide hydrolases and their application in organic synthesis. In R N Patel (ed) Green biocatalysis. Wiley, New York, pp 210–216
Bala N, Chimni SS (2010) Recent developments in the asymmetric hydrolytic ring opening of epoxides catalysed by microbial epoxide hydrolase. Tetrahedron-Asymmetry 21(24):2879–2898. https://doi.org/10.1016/j.tetasy.2010.11.013
Barth S, Fischer M, Schmid RD, Pleiss J (2004) Sequence and structure of epoxide hydrolases: a systematic analysis. Proteins 55(4):846–855. https://doi.org/10.1002/prot.20013
Basith S, Manavalan B, Lee G, Kim SG, Choi S (2011) Toll-like receptor modulators: a patent review (2006–2010). Expert Opin Ther Patents 21(6):927–944. https://doi.org/10.1517/13543776.2011.569494
Bisi A, Rampa A, Budriesi R, Gobbi S, Belluti F, Ioan P, Valoti E, Chiarini A, Valenti P (2003) Cardiovascular hybrid drugs: new benzazepinone derivatives as bradycardic agents endowed with selective β1-non-competitive antagonism. Bioorg Med Chem 11(7):1353–1361. https://doi.org/10.1016/s0968-0896(02)00621-1
Botes AL, Weijers CAGM, Van Dyk MS (1998) Biocatalytic resolution of 1,2-epoxyoctane using resting cells of different yeast strains with novel epoxide hydrolase activities. Biotechnol Lett 20(4):421–426
Cambell D, Duron SG (2013) Preparation of 8-ethyl-6-(aryl)-pyrido[2,3-d]pyrimidin-7 (8H)-one compds. as PAK inhibitors useful in treatment of nervous system disorders and cancer. U.S. Patent Appl 2013043232
Choi WJ, Choi CY, De Bont JAM, Weijers CAGM (2000) Continuous production of enantiopure 1,2-epoxyhexane by yeast epoxide hydrolase in a two-phase membrane bioreactor. Appl Microbiol Biotechnol 54(5):641–646. https://doi.org/10.1007/s002530000451
De Carvalho CC (2011) Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv 29(1):75–83. https://doi.org/10.1016/j.biotechadv.2010.09.001
Deregnaucourt J, Archelas A, Barbirato F, Paris J-M, Furstoss R (2007) Enzymatic transformations 63. High-concentration two liquid-liquid phase Aspergillus niger epoxide hydrolase-catalysed resolution: application to trifluoromethyl-substituted aromatic epoxides. Adv Synth Catal 349(8–9):1405–1417. https://doi.org/10.1002/adsc.200700085
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol 50(3):1351–1371. https://doi.org/10.1099/00207713-50-3-1351
Gao P, Wu S, Praveen P, Loh KC, Li Z (2017) Enhancing productivity for cascade biotransformation of styrene to (S)-vicinal diol with biphasic system in hollow fiber membrane bioreactor. Appl Microbiol Biotechnol 101(5):1857–1868. https://doi.org/10.1007/s00253-016-7954-1
Hu D, Tang C, Li C, Kan T, Shi X, Feng L, Wu M (2017) Stereoselective hydrolysis of epoxides by reVrEH3, a Novel Vigna radiata epoxide hydrolase with high enantioselectivity or high and complementary regioselectivity. J Agric Food Chem 65(45):9861–9870. https://doi.org/10.1021/acs.jafc.7b03804
Hwang S, Choi CY, Lee EY (2010) Bio- and chemo-catalytic preparations of chiral epoxides. J Ind Eng Chem 16(1):1–6. https://doi.org/10.1016/j.jiec.2010.01.001
Kotik M, Kyslík P (2006) Purification and characterisation of a novel enantioselective epoxide hydrolase from Aspergillus niger M200. Biochim Biophys Acta-Gen Subj 1760(2):245–252. https://doi.org/10.1016/j.bbagen.2005.11.002
Kotik M, Štěpánek V, Grulich M, Kyslík P, Archelas A (2010) Access to enantiopure aromatic epoxides and diols using epoxide hydrolases derived from total biofilter DNA. J Mol Catal B-Enzym 65(1–4):41–48. https://doi.org/10.1016/j.molcatb.2010.01.016
Kotik M, Archelas A, Wohlgemuth R (2012) Epoxide hydrolases and their application in organic synthesis. Curr Org Chem 16(4):451–482. https://doi.org/10.2174/138527212799499840
Li C, Hu D, Zong X-C, Deng C, Feng L, Wu M-C, Li J-F (2017) Asymmetric hydrolysis of styrene oxide by Pv EH2, a novel Phaseolus vulgaris epoxide hydrolase with extremely high enantioselectivity and regioselectivity. Catal Commun 102:57–61. https://doi.org/10.1016/j.catcom.2017.08.026
Li F-L, Kong X-D, Chen Q, Zheng Y-C, Xu Q, Chen F-F, Fan L-Q, Lin G-Q, Zhou J, Yu H-L, Xu J-H (2018) Regioselectivity engineering of epoxide hydrolase: near-perfect enantioconvergence through a single site mutation. ACS Catal 8(9):8314–8317. https://doi.org/10.1021/acscatal.8b02622
Lin H, Liu J-Y, Wang H-B, Ahmed AAQ, Wu Z-L (2011) Biocatalysis as an alternative for the production of chiral epoxides: a comparative review. J Mol Catal B-Enzym 72(3–4):77–89. https://doi.org/10.1016/j.molcatb.2011.07.012
Lind MES, Himo F (2016) Quantum chemical modeling of enantioconvergency in soluble epoxide hydrolase. ACS Catal 6(12):8145–8155. https://doi.org/10.1021/acscatal.6b01562
Matsumoto M, Sugimoto T, Ishiguro Y, Yamaguchi H, Kondo K (2014) Effect of organic solvents and ionic liquids on resolution of 2-epoxyhexane by whole cells of Rhodotorula glutinis in a two-liquid phase system. J Chem Technol Biotechnol 89(4):522–527. https://doi.org/10.1002/jctb.4148
Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL (2010) Discovery of a proneurogenic, neuroprotective chemical. Cell 142(1):39–51. https://doi.org/10.1016/j.cell.2010.06.018
Reetz MT, Bocola M, Wang L-W, Sanchis J, Cronin A, Arand M, Zou J, Archelas A, Bottalla A-L, Naworyta A, Mowbray SL (2009) Directed evolution of an enantioselective epoxide hydrolase: uncovering the source of enantioselectivity at each evolutionary stage. J Am Chem Soc 131(21):7334–7343. https://doi.org/10.1021/ja809673d
Serrano-Hervas E, Garcia-Borras M, Osuna S (2017) Exploring the origins of selectivity in soluble epoxide hydrolase from Bacillus megaterium. Org Biomol Chem 15(41):8827–8835. https://doi.org/10.1039/c7ob01847a
Stephenson KA, Wilson AA, Meyer JH, Houle S, Vasdev N (2008) Facile radiosynthesis of fluorine-18 labeled β-blockers. Synthesis, radiolabeling, and ex vivo biodistribution of [18F]-(2S and 2R)-1-(1-fluoropropan-2-ylamino)-3-(m-tolyloxy)propan-2-ol. J Med Chem 51(16):5093–5100. https://doi.org/10.1021/jm800227h
Sun Z, Lonsdale R, Wu L, Li G, Li A, Wang J, Zhou J, Reetz MT (2016) Structure-guided triple-code saturation mutagenesis: efficient tuning of the stereoselectivity of an epoxide hydrolase. ACS Catal 6(3):1590–1597. https://doi.org/10.1021/acscatal.5b02751
Visser H, Weijers CAGM, van Ooyen AJJ, Verdoes JC (2002) Cloning, characterization and heterologous expression of epoxide hydrolase-encoding cDNA sequences from yeasts belonging to the genera Rhodotorula and Rhodosporidium. Biotechnol Lett 24(20):1687–1694. https://doi.org/10.1023/a:1020613803342
Weijers CAGM (1997) Enantioselective hydrolysis of aryl, alicyclic and aliphatic epoxides by Rhodotorula glutinis. Tetrahedron-Asymmetry 8(4):639–647. https://doi.org/10.1016/S0957-4166(97)00012-8
Weijers CAGM, Botes AL, van Dyk MS, de Bont JAM (1998) Enantioselective hydrolysis of unbranched aliphatic 1,2-epoxides by Rhodotorula glutinis. Tetrahedron-Asymmetry 9(3):467–473. https://doi.org/10.1016/S0957-4166(97)00639-3
Woo JH, Kang JH, Hwang YO, Cho JC, Kim SJ, Kang SG (2010) Biocatalytic resolution of glycidyl phenyl ether using a novel epoxide hydrolase from a marine bacterium, Maritimibacter alkaliphilus KCCM 42376. J Biosci Bioeng 109(6):539–544. https://doi.org/10.1016/j.jbiosc.2009.11.019
Wu S, Li A, Chin YS, Li Z (2013) Enantioselective hydrolysis of racemic and meso-epoxides with recombinant Escherichia coli expressing epoxide hydrolase from Sphingomonas sp. HXN-200: preparation of epoxides and vicinal diols in high ee and high concentration. ACS Catal 3(4):752–759. https://doi.org/10.1021/cs300804v
Xu Y, Xu J-H, Pan J, Tang Y-F (2004) Biocatalytic resolution of glycidyl aryl ethers by Trichosporon loubierii: cell/substrate ratio influences the optical purity of (R)-epoxides. Biotechnol Lett 26(15):1217–1221. https://doi.org/10.1023/B:BILE.0000036598.35494.de
Yoo SS, Park S, Lee EY (2008) Enantioselective resolution of racemic styrene oxide at high concentration using recombinant Pichia pastoris expressing epoxide hydrolase of Rhodotorula glutinis in the presence of surfactant and glycerol. Biotechnol Lett 30(10):1807–1810. https://doi.org/10.1007/s10529-008-9762-x
Zhao J, Chu Y-Y, Li A-T, Ju X, Kong X-D, Pan J, Tang Y, Xu J-H (2011) An unusual (R)-selective epoxide hydrolase with high activity for facile preparation of enantiopure glycidyl ethers. Adv Synth Catal 353(9):1510–1518. https://doi.org/10.1002/adsc.201100031
Zheng GW, Xu JH (2011) New opportunities for biocatalysis: driving the synthesis of chiral chemicals. Curr Opin Biotechnol 22(6):784–792. https://doi.org/10.1016/j.copbio.2011.07.002
Zhu QQ, He WH, Kong XD, Fan LQ, Zhao J, Li SX, Xu JH (2014) Heterologous overexpression of Vigna radiata epoxide hydrolase in Escherichia coli and its catalytic performance in enantioconvergent hydrolysis of p-nitrostyrene oxide into (R)-p-nitrophenyl glycol. Appl Microbiol Biotechnol 98(1):207–218. https://doi.org/10.1007/s00253-013-4845-6
Zou SP, Zheng YG, Wu Q, Wang ZC, Xue YP, Liu ZQ (2018) Enhanced catalytic efficiency and enantioselectivity of epoxide hydrolase from Agrobacterium radiobacter AD1 by iterative saturation mutagenesis for (R)-epichlorohydrin synthesis. Appl Microbiol Biotechnol 102(2):733–742. https://doi.org/10.1007/s00253-017-8634-5
Acknowledgments
The authors are grateful to Prof. Xianzhang Wu (School of Biotechnology, Jiangnan University, Jiangsu, China) for providing technical assistance.
Funding
This work was financially supported by the China Postdoctoral Science Foundation (No. 2018M630522) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_1804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 710 kb)
Rights and permissions
About this article
Cite this article
Xu, XF., Hu, D., Hu, BC. et al. Near-perfect kinetic resolution of o-methylphenyl glycidyl ether by RpEH, a novel epoxide hydrolase from Rhodotorula paludigena JNU001 with high stereoselectivity. Appl Microbiol Biotechnol 104, 6199–6210 (2020). https://doi.org/10.1007/s00253-020-10694-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10694-w