Abstract
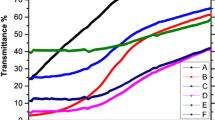
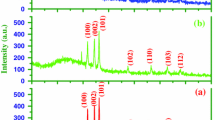
Nanostructured manganese oxide (Mn2O3) thin films were synthesized by spin coating method. The effect of preparation conditions, such as calcination temperature, rotation speed, as well as solution aging time on structural, morphological, and optical properties of the samples were investigated. The phase of the grown films changed from amorphous to orthorhombic by applying the calcination temperature beyond 400 °C. At low calcination temperature, agglomerations of the particles happened and no uniform structure formed. Furthermore, the results indicated that employing higher rotation speeds led to decrement of the samples crystallinity. Aging the solution did not change the absorption edge of the samples. However, the optical transmittance of the samples decreased by about 10 percent.

Surface FESEM image of seven layer Mn2O3 thin film calcined at 500 °C
Highlights
-
Mn2O3 thin films have been prepared by sol-gel spin coating technique.
-
Structural, morphological, and optical properties of Mn2O3 thin films have been studied.
-
The effects of calcination temperature as well as rotation spin in spinning process have been investigated.
-
The effect of solution aging on physical properties of the Manganese oxide thin films has been studied.










Similar content being viewed by others

References
Shaikh A, Waikar M, Sonkawade R (2019) Effect of different precursors on electrochemical properties of manganese oxide thin films prepared by SILAR method. Synth Met 247:1–9
Astinchap B, Moradian R, Namdari T, Jurečka S, Ţălu Ş (2019) Prepared σ-MnO2 thin films by chemical bath deposition methods and study of its optical and microstructure properties. Optical Quantum Electron 51(6):170
Ahmadian H, Tehrani FS, Aliannezhadi M (2019) Hydrothermal synthesis and characterization of WO3 nanostructures: effects of capping agent and pH. Mater Res Express 6(10):105024
Jamali M, Tehrani FS (2020) Effect of synthesis route on the structural and morphological properties of WO3 nanostructures. Mater Sci Semiconductor Process 107:104829
Sharma S, Chauhan P, Husain S (2016) Structural and optical properties of Mn2O3 nanoparticles and its gas sensing properties. Adv Mater Proc 1:220–225
Mansouri M (2018) Effects of vacancy-defected, dopant and the adsorption of water upon Mn2O3 and Mn3O4 (001) surfaces: a first-principles study. Acta Physica Polonica A 133(5):1178–1185
Yang S et al. (2019) The excellent electrochemical performances of ZnMn2O4/Mn2O3: The composite cathode material for potential aqueous zinc ion batteries. J Electroanalytical Chem 832:69–74
Parveen N et al. (2019) Feasibility of using hollow double walled Mn2O3 nanocubes for hybrid Na-air battery. Chem Eng J 360:415–422
Abrego-Martínez J et al. (2019) Nanostructured Mn2O3/Pt/CNTs selective electrode for oxygen reduction reaction and methanol tolerance in mixed-reactant membraneless micro-DMFC. Electrochim Acta 297:230–239
Lee JH, Kim KJ (2013) Superior electrochemical properties of porous Mn2O3-coated LiMn2O4 thin-film cathodes for Li-ion microbatteries. Electrochim Acta 102:196–201
Sasikumar R, Chen T-W, Chen S-M, Rwei S-P, Yu M-C(2018) Facile synthesis of Mn2O3 for highly active catalytic oxidation and reduction of organic substances and electrochemical determination of L-methionine Int J Electrochem Sci 13(5):4561–4574
Korotcenkov G, Brinzari V, Ham M (2018) Materials acceptable for gas sensor design: advantages and limitations. Key Eng Mater 780:80–89
Hou Y, Cheng Y, Hobson T, Liu J (2010) Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett 10(7):2727–2733
Liu P-P, Zheng Y-Q, Zhu H-L, Li T-T (2019) Mn2O3 hollow nanotube arrays on Ni foam as efficient supercapacitors and electrocatalysts for oxygen evolution reaction. ACS Appl Nano Mater 2(2):744–749
Zhang X et al. (2019) Influence of hydrothermal synthesis temperature on the redox and oxygen mobility properties of manganese oxides in the catalytic oxidation of toluene. Trans Metal Chem 44:663–670
Bach S, Henry M, Baffier N, Livage J (1990) Sol-gel synthesis of manganese oxides. J Solid State Chem 88(2):325–333
Yang D (2012) Pulsed laser deposition of cobalt-doped manganese oxide thin films for supercapacitor applications. J Power Sources 198:416–422
Broughton J, Brett M (2004) Investigation of thin sputtered Mn films for electrochemical capacitors. Electrochim Acta 49(25):4439–4446
Maruyama T, Osaki Y (1995) Electrochromic properries of manganese oxide thin films prepared by chemical vapor deposition. J Electrochem Soc 142(9):3137–3141
Walter C, Menezes PW, Loos S, Dau H, Driess M (2018) Facile formation of nanostructured manganese oxide films as high‐performance catalysts for the oxygen evolution reaction. ChemSusChem 11(15):2554–2561
Sarkar A, Satpati AK, Kumar V, Kumar S (2015) Sol-gel synthesis of manganese oxide films and their predominant electrochemical properties. Electrochim Acta 167:126–131
Nilsen O, Fjellvåg H, Kjekshus A (2003) Growth of manganese oxide thin films by atomic layer deposition. Thin Solid Films 444(1–2):44–51
Komaraiah D, Radha E, Reddy MR, Kumar JS, Sayanna R (2019) Structural, optical properties and photocatalytic activity of nanocrystalline TiO2 thin films deposited by sol–gel spin coating. i-Manager’s J Mater Sci 7(1):28
Ziller S, von Bülow J, Dahl S, Lindén M (2017) A fast sol–gel synthesis leading to highly crystalline birnessites under non-hydrothermal conditions. Dalton Trans 46(14):4582–4588
Sahu N, Parija B, Panigrahi S (2009) Fundamental understanding and modeling of spin coating process: a review. Indian J Phys 83(4):493–502
Maezono R, Ishihara S, Nagaosa N (1998) Phase diagram of manganese oxides. Phys Rev B 58(17):11583
Devaraj S, Munichandraiah N (2008) Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112(11):4406–4417
Awad MA, Hadia NMA (2018) Towards understanding the morphological, magnetic, optical and electrical properties of MnO2 nanowires for magneto-and optoelectronic applications. J Mater Sci: Mater Electron 29(24):20695–20702
Augustin M et al. (2015) Manganese oxide phases and morphologies: a study on calcination temperature and atmospheric dependence. Beilstein J Nanotechnol 6(1):47–59
Fang Y, Wang Y, Wang F, Zhu J (2019) 3D structured Mn2O3 synthesized using tween surfactant: influence on the morphology and oxygen reduction catalytic performance. CrystEngComm 21(3):420–429
Falahatgar S, Ghodsi F (2016) Optical characterization of nanostructured MnO2ZnO thin films prepared from acetate-based sol−gel precursors. Opt-Int J Light Electron Opt 127(3):1059–1065
Chin S-F, Pang S-C, Anderson MA (2002) Material and electrochemical characterization of tetrapropylammonium manganese oxide thin films as novel electrode materials for electrochemical capacitors. J Electrochem Soc 149(4):A379–A384
Fau P, Bonino J, Rousset A (1994) Electrical properties of sputtered MnO2 thin films. Appl Surf Sci 78(2):203–210
Pang SC, Chin SF, Ling CY (2011) Preparation and characterization of self-assembled manganese dioxide thin films. J Nanotechnol 2011:789305
Hernández S et al. (2016) Spin-coated vs. electrodeposited Mn oxide films as water oxidation catalysts. Materials 9(4):296
Hassanien A, Akl AA (2016) Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattice Microst 89:153–169
Aawani E, Memarian N, Dizaji HR (2019) Synthesis and characterization of reduced graphene oxide–V2O5 nanocomposite for enhanced photocatalytic activity under different types of irradiation. J Phys Chem Solids 125:8–15
Venkatramu V et al. (2010) Nanocrystalline lanthanide-doped Lu3Ga5O12 garnets: interesting materials for light-emitting devices. Nanotechnology 21(17):175703
Sain S, Patra S, Pradhan S (2012) Quickest ever single-step mechanosynthesis of Cd0.5Zn0.5S quantum dots: nanostructure and optical characterizations. Mater Res Bull 47(4):1062–1072
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bahadori, A., Dizaji, H.R., Memarian, N. et al. Effect of preparation conditions on physical properties of manganese oxide thin films. J Sol-Gel Sci Technol 95, 180–189 (2020). https://doi.org/10.1007/s10971-020-05296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05296-x