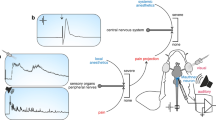
Abstract
Tricaine mesylate, also known as MS-222, was investigated to characterize its effects on sensory neurons, synaptic transmission at the neuromuscular junction, and heart rate in invertebrates. Three species were examined: Drosophila melanogaster, blue crab (Callinectes sapidus), and red swamp crayfish (Procambarus clarkii). Intracellular measures of action potentials in motor neurons of the crayfish demonstrated that MS-222 dampened the amplitude, suggesting that voltage-gated Na + channels are blocked by MS-222. This is likely the mechanism behind the reduced activity measured in sensory neurons and depressed synaptic transmission in all three species as well as reduced cardiac function in the larval Drosophila. To address public access to data, a group effort was used for analysis of given data sets, blind to the experimental design, to gauge analytical accuracy. The determination of a threshold in analysis for measuring extracellular recorded sensory events is critical and is not easily performed with commercial software.
Graphic abstract









Similar content being viewed by others
References
Alexandrowicz JS (1967) Receptor organs in the coxal region of Palinurus vulgaris. J Marine Biol Assoc UK 47:415–432
Applegate JR Jr, Dombrowski DS, Christian LS, Bayer MP, Harms CA, Lewbart GA (2016) Tricaine methanesulfonate (ms-222) sedation and anesthesia in the purple-spined sea urchin (Arbacia punctulata). J Zoo Wildlife Med 47(4):1025–1033. https://doi.org/10.1638/2015-0288.1
Archibald KE, Scott GN, Bailey KM, Harms CA (2019) 2-phenoxyethanol (2-pe) and tricaine methanesulfonate (ms-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). J Zoo Wildl Med 50(1):96–106. https://doi.org/10.1638/2018-0085
Attili S, Hughes SM (2014) Anaesthetic tricaine acts preferentially on neural voltage-gated sodium channels and fails to block directly evoked muscle contraction. PLoS ONE 9(8):e103751. https://doi.org/10.1371/journal.pone.0103751
Atwood HL, Cooper RL (1996) Assessing ultrastructure of crustacean and insect neuromuscular junctions. J Neurosci Methods 69:51–58
Bagnéris C, DeCaen PG, Naylor CE, Pryde DC, Nobeli I, Clapham DE, Wallace BA (2014) Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism. Proc Natl Acad Sci USA 111:8428–8433
Baierlein B, Thurow AL, Atwood HL, Cooper RL (2011) Membrane potentials, synaptic responses, neuronal circuitry, neuromodulation and muscle histology using the crayfish: student laboratory exercises. J Vis Exp 47, http://www.jove.com/Details.php?ID=2322. https://doi.org/10.3791/2325
Bakshi A, Patrick LE, Wischusen EW (2016) A framework for implementing course-based undergraduate research experiences (CUREs) in freshman biology labs. Am Biol Teach 78(6):448–455
Barron AB (2000) Anaesthetising for behavioural studies. J Insect Physiol 46(4):439–442
Bewick GS, Banks RW (2015) Mechanotransduction in the muscle spindle. Pflügers Archiv 467(1):175–190. https://doi.org/10.1007/s00424-014-1536-9
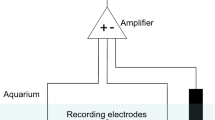
Bierbower SM, Cooper RL (2009) Measures of heart and ventilatory rates in freely moving crayfish. J Vis Exp 32, http://www.jove.com/index/details.stp?id=1594. https://doi.org/10.3791/1594
Bowker JD, Trushenski JT, Glover DC, Carty DG, Wandelear N (2014) Sedative options for fish research: a brief review with new data on sedation of warm-, cool-, and coldwater fishes and recommendations for the drug approval process. Rev Fish Biol Fish 25(1):147–163. https://doi.org/10.1007/s11160-014-9374-6
Brodbelt DC (2009) Perioperative mortality in small animal anaesthesia. Veterinary J (Lond) 182:152–161
Brown PB, White MR, Chaille J, Russell M, Oseto C (1996) Evaluation of three anesthetic agents for crayfish (Orconectes virilis). J Shellfish Res 15(2):433–435
Butterworth JFT, Strichartz GR (1990) Molecular mechanisms of local anesthesia: a review. Anesthesiology 72:711–734
Cakir Y, Strauch SM (2005) Tricaine (MS-222) is a safe anesthetic compound compared to benzocaine and pentobarbital to induce anesthesia in leopard frogs (Rana pipiens). Pharmacol Rep 57:467–474
Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon 49:124–141
Clare JDJ, Townsend PA, Anhalt-Depies C, Locke C, Stenglein JL, Frett S, Martin KJ, Singh A, Van Deelen TR, Zuckerberg B (2019) Making inference with messy (citizen science) data: when are data accurate enough and how can they be improved? Ecol Appl 29(2):e01849. https://doi.org/10.1002/eap.1849
Collymore C, Banks EK, Turner PV (2016) Lidocaine hydrochloride compared with ms222 for the euthanasia of zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 55(6):816–820
Cooper RL (2008) Mapping proprioceptive neurons on chordotonal organs in the crab. Cancer magister Crustaceana 81(4):447–475
Cooper AS, Cooper RL (2009). Historical view and demonstration of physiology at the NMJ at the crayfish opener muscle. J Vis Exp 33. http://www.jove.com/index/details.stp?id=1595. https://doi.org/10.3791/1595
Cooper RL, Govind CK (1991) Axon composition of the proprioceptive PD nerve during growth and regeneration of lobster claws. J Exp Zool 260:181–193
Cooper RL, Hartman HB (1999) Quantification of responses from proprioceptive neurons in the limbs of the crab, Cancer magister. J Exp Zool 284:629–636
Cooper AS, Rymond KE, Ward MA, Bocook EL, Cooper RL (2009) Monitoring heart function in larval Drosophila melanogaster for physiological studies. J Vis Exp 32, http://www.jove.com/index/details.stp?id=1596
Cooper AS, Leksrisawat B, Mercier AJ, Gilberts AB, Cooper RL (2011) Physiological experimentations with the crayfish hindgut. J Vis Exp 47, http://www.jove.com/details.php?id=2324doi: https://doi.org/10.3791/2324
Coyle SD, Dasgupta S, Tidwell JH, Beavers T, Bright LA, Yasharian DK (2005) Comparative Efficacy of Anesthetics for the Freshwater Prawn Macrobrachiurn rosenbergii. J World Aquac Soc 36:282–290. https://doi.org/10.1111/j.1749-7345.2005.tb00332.x
Crider ME, Cooper RL (2000) Differential facilitation of high- and low-output nerve terminals from a single motor neuron. J Appl Physiol 88:987–996
Davies TG, Field LM, Usherwood PN, Williamson MS (2007) A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species. Insect Mol Biol 16:361–375
Davis DJ, Klug J, Hankins M, Doerr HM, Monticelli SR, Song A, Gillespie CH, Bryda EC (2015) Effects of clove oil as a euthanasia agent on blood collection efficiency and serum cortisol levels in Danio rerio. J Am Assoc Lab Anim Sci 54(5):564–567
DayaramV, Malloy C, Martha S, Alvarez B, Chukwudolue I, Dabbain N, Mahmood DD, Goleva S, Hickey T, Ho A, King M, Kington P, Mattingly M, Potter S, Simpson L, Spence A, Uradu H, Van Doorn JL, Cooper RL (2017) Stretch activated channels in propriocep-tive chordotonal organs of crab and crayfish are sensitive to Gd3 + but not amiloride, ruthenium red or low pH. IMPLUSE The Premier Undergraduate Neuroscience Journal, https://impulse.appstate.edu/issues/2017
de Castro C, Titlow J, Majeed ZR, Cooper RL (2014) Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J Comp Physiol A 200:83–92
de Castro C, Titlow JS, Majeed ZR, Malloy C, King KE, Cooper RL (2019) Chemical and mechanical factors required for maintaining cardiac rhythm in Drosophila melanogaster larva. J Entomol 16(2):62–73
Desai-Shah M, Viele K, Sparks G, Nadolski J, Hayden B, Srinivasan VK, Cooper RL (2008) Assessment of synaptic function during short-term facilitation in motor nerve terminals in the crayfish. Open Neurosci J 2:24–35
Desai-Shah M, Papoy AR, Ward M, Cooper RL (2010) Roles of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger in regulation of heart rate in larval Drosophila. Open Physiol J 3:16–36
Diggles K (2018) Review of some scientific issues related to crustacean welfare. International Council for the Exploration of the Sea Journal of Marine Science. fsy058, https://doi.org/10.1093/icesjms/fsy058
EFSA (2005). Aspects of the biology and welfare of animals used for experimental and other scientific purposes. EFSA J 292: 1–46. https://ec.europa.eu/environment/chemicals/lab_animals/pdf/efsa_opinion.pdf
Flecknell PA (1984) The relief of pain in laboratory animals. Lab Anim 18(2):147–160. https://doi.org/10.1258/002367784780891226
Frazier DT, Narahashi T (1975) Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol 33(2):313–317
Gaynor JS, Dunlop CI, Wagner AE, Wertz EM, Golden AE, Demme WC (1999) Complications and mortality associated with anesthesia in dogs and cats. J Am Anim Hosp Assoc 35(1):13–17. https://doi.org/10.5326/15473317-35-1-13
Gilbert PW, Wood FG (1957) Method of anesthetizing large sharks and rays safely and rapidly. Science 126(3266):212–213. https://doi.org/10.1126/science.126.3266.212
Gu GG, Singh S (1995) Pharmacological analysis of heartbeat in Drosophila. J Neurobiol 28:269–280
Guttman D (1967) MS-222 Sandoz as an anesthetic for black fly larvae (Diptera: Simuliidae). J Med Entomol 4(4):477–478
Hartman HB, Boettiger EG (1967) The functional organization of the propus-dactylus organ in Cancer irroratus Say. Comp Biochem Physiol 22:651–663
Hartman HB, Cooper RL (1994) Regeneration and molting effects on a proprioceptor organ in the Dungeness crab, Cancer magister. J Neurobiol 25:461–471
Håstein T, Scarfe AD, Lund VL (2005) Science-based assessment of welfare: aquatic animals. Revue scientifique et technique (International Office of Epizootics) 24(2):529–547
He P, Southard RC, Whiteheart SW, Cooper RL (1999) Role of alpha-SNAP in promoting efficient neurotransmission at the crayfish neuromuscular junction. J Neurophysiol 82:3406–3416
Holsinger RC, Cooper RL (2012) Effect of environment and modulators on hindgut and heart function in invertebrates: Crustaceans and Drosophila. Tested Studies for Laboratory Teaching, Volume 33 (K. McMahon, Editor). Proceedings of the 33rd Conference of the Association for Biology Laboratory Education (ABLE). http://www.ableweb.org/volumes/vol-33/v33reprint.php?ch=7
Javahery S, Nekoubin H, Moradlu AH (2012) Effect of anaesthesia with clove oil in fish. Fish Physiol Biochem 38:1545–1552
Johnson BR, Wyttenbach RA, Hoy RR (2014) Crustaceans as model systems for teaching neuroscience: past, present, and future. In: Derby C, Thiel M (eds) Nervous systems and control of behavior, the natural history of the Crustacea. Oxford University Press, New York
Kuffler SW (1954) Mechanisms of activation and motor control of stretch receptors in lobster and crayfish. J Neurophysiol 17:558–574
Kurdyak P, Atwood HL, Stewart BA, Wu C-F (1994) Differential physiology and morphology of motor axons to ventral longitudinal muscle in larval Drosophila. J Comp Neurol 350:463–472
Lea T (1996) Caffeine and micromolar Ca2 + concentrations can release Ca2 + from ryanodine-sensitive stores in crab and lobster striated muscle fibres. J Exp Biol 199(11):2419–2428
Lea F, Malheiro B, González-Vélez H, Burguillo JC (2017) Trust-based modelling of multi-criteria crowdsourced data. Data Sci Eng 2(3):199–209
Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, Lee JH, Kim JS, Oh SB (2005) Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res 84:848–851
Lee S, Goodchild SJ, Ahern CA (2012) Local anesthetic inhibition of a bacterial sodium channel. J Gen Physiol 139:507–516
Leksrisawat B, Cooper AS, Gilberts AB, Cooper RL (2010) Response properties of muscle receptor organs in the crayfish abdomen: A student laboratory exercise in proprioception. J Vis Exp 45 http://www.jove.com/index/details.stp?id=2323. https://doi.org/10.3791/2323
Li H, Harrison D, Jones G, Jones D, Cooper RL (2001) Alterations in development, behavior, and physiology in Drosophila larva that have reduced ecdysone production. J Neurophysiol 85:98–104
Lierz M, Korbel R (2012) Anesthesia and analgesia in birds. J Exotic Pet Med 21(1):44–58. https://doi.org/10.1053/j.jepm.2011.11.008
Linn MC, Palmer E, Baranger A, Gerard E, Stone E (2015) Undergraduate research experiences: impacts and opportunities. Science 347:1261757
Listerman L, Deskins J, Bradacs H, Cooper RL (2000) Measures of heart rate during social interactions in crayfish and effects of 5-HT. Compa Biochem Physiol A 125:251–264
Majeed ZR, Nichols CD, Cooper RL (2013a) 5-HT stimulation of heart rate in Drosophila does not act through cAMP as revealed by pharmacogenetics. J Appl Physiol 115:1656–1665
Majeed ZR, Titlow J, Hartman HB, Cooper RL (2013b) Proprioception and tension receptors in crab limbs: student laboratory exercises. J Vis Exp 80:e51050. https://doi.org/10.3791/51050
Majeed ZR, Stacy A, Cooper RL (2014) Pharmacological and genetic identification of serotonin receptor subtypes on Drosophila larval heart and aorta. J Comp Physiol B 184:205–219
Majeed Z, Koch F, Morgan J, Anderson H, Wilson J, Cooper RL (2017) A novel educa-tional module to teach neural circuits for college and high school students: NGSS-neurons, genetics, and selective stimulations. F1000Research: Immediate & Transparent Publishing for Life Scientists. F1000 Research Ltd, Middlesex House, 34-42 Cleveland St, London W1T 4LB, UK.https://f1000research.com/articles/6-117/v1
Malloy CA, Ritter K, Robinson J, English C, Cooper RL (2015) Pharmacological identification of cholinergic receptor subtypes on Drosophila melanogaster larval heart. J Comp Physiol B 186:45–57
Malloy C, DayaramV Martha S, Alvarez B, Chukwudolue I, Dabbain N, Mahmood DD, Goleva S, Hickey T, Ho A, King M, Kington P, Mattingly M, Potter S, Simpson L, Spence A, Uradu H, Van Doorn JL, Weineck K, Cooper RL (2017) The effects of potassium and muscle homogenate on proprioceptive responses in crayfish and crab. J Exp Zool 327(6):366–379
Mendelson M (1963) Some factors in the activation of crab movement receptors. J Exp Biol 40:157–169
Ramlochansingh C, Branoner F, Chagnaud BP, Straka H (2014) Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PLoS ONE 9(7):e101606. https://doi.org/10.1371/journal.pone.0101606
Robinson MM, Martin JM, Atwood HL, Cooper RL (2011). Modeling biological membranes with circuit boards and measuring conduction velocity in axons: Student laboratory exercises. J Vis Exp 47, http://www.jove.com/details.php?id=2325. https://doi.org/10.3791/2325
Roth B, Øines S (2010) Stunning and killing of edible crabs (Cancer pagurus). Anim Welf 19:287–294
Rydqvist B, Lin JH, Sand P, Swerup C (2007) Mechanotransduction and the crayfish stretch receptor. Physiol Behav 92(1–2):21–28
Schmit O, Mezquita F (2010) Experimental test on the use of MS-222 for ostracod anaesthesia: concentration, immersion period and recovery time. J Limnol 69(2):350–352. https://doi.org/10.3274/JL10-69-2-N1
Sénatore S, Reddy VR, Sémériva M, Perrin L, Lalevée N (2010) Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet 6(9):e1001088. https://doi.org/10.1371/journal.pgen.1001088
SOFIA 2018 http://www.fao.org/publications/sofia/en/ (Accessed on Aug. 20, 2019)
Sømme LS (2005) Sentience and pain in invertebrates. Report to Norwegian Scientific Committee for Food Safety. Norwegian University of Life Sciences, Oslo. Website: vkm.no/dav/0327284150.pdf
Stanback M, Stanback AE, Akhtar S, Basham R, Chithrala B, Collis B, Heberle BA, Higgins E, Lane A, Marella S, Ponder M, Raichur P, Silverstein A, Stanley C, Vela K, Cooper RL (2019). The effect of lipopolysaccharides on primary sensory neurons in crustacean models. IMPLUSE https://impulse.appstate.edu/articles/2019/effect-lipopolysaccharides-primary-sensory-neurons-crustacean-models
Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF (1994) Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J Comp Physiol A 175:179–191
Stoskopf MK (2006) Coelenterates. In: Lewbart GA (ed) Invertebrate medicine, 1st edn. Blackwell Publishing, Ames, pp 19–52
Thenappan A, Dupont-Versteegden EE, Cooper RL (2019) An educational model for understanding acute deep tissue injury of motor units. Journal of Young Investigators 36(5):62–71
Titlow J, Majeed ZR, Hartman HB, Burns E, Cooper RL (2013a) Neural circuit recording from an intact cockroach nervous system. J Vis Exp 80:e51050. https://doi.org/10.3791/51050
Titlow JS, Rufer JM, King KE, Cooper RL (2013b) Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol Rep 1(2):e00020. https://doi.org/10.1002/phy2.20
Wayson KA (1976) Studies on the comparative pharmacology and selective toxicity of tricaine methanesulfonate: metabolism as a basis of the selectivity toxicity in poikilotherms. J Pharmacol Exp Ther 198(3):695–708
Weineck K, Ray AJ, Fleckenstein L, Medley M, Dzubuk N, Piana E, Cooper RL (2018) Physiological changes as a measure of crustacean welfare under different standardized stunning techniques: cooling and electroshock. Animals 8(9):e158. https://doi.org/10.3390/ani8090158
Weineck K, Stanback AE, Cooper RL (2019) The effects of eugenol as an anesthetic for an insect: Drosophila, adult, larval heart rate and synaptic transmission. Article 54. In: McMahon K editor. Tested studies for laboratory teaching. Volume 40. Proceedings of the 39th Conference of the Association for Biology Laboratory Education (ABLE). http://www.ableweb.org/volumes/vol-40/?art=# Vol 40, Article 54, 2019
Whitear M (1960) Chordotonal organs in Crustacea. Nature, London 187:522–523
Whitear M (1962) The fine structure of crustacean proprioceptors. I. The chordotonal organs in the legs of the shore crab, Carcinus meanas. Philos TransR Soc B 245:291–325
Whitear M (1965) The fine structure of crustacean proprioceptors. II. The thoracico- -coxal organs in Carcinus, Pagurus and Astacus. Philos TransR Soc B 248(752):437–462
Wiersma CAG (1959) Movement receptors in decapod Crustacea. J Marine Biol Assoc UK 38:143–152
Wiersma CAG, Boettiger EG (1959) Unidirectional movement fibers from a proprioceptive organ of the crab, Carcinus maenas. J Exp Biol 36:102–112
Wilkens JL, Mercier AJ, Evans J (1985) Cardiac and ventilatory responses to stress and to neurohormonal modulators by the shore crab Carcinus maenas. Comp Biochem Physiol 82C:337–343
Wycoff S, Weineck K, Conlin S, Grau E, Bradley A, Cantrell D, Eversole S, Grachen C, Hall K, Hawthorne D, Kinmon C, Ortiz Guerrero P, Patel B, Samuels K, Suryadevara C, Valdes G, Ray A, Fleckenstein L, Piana E, Cooper RL (2018) Investigating potential effects of clove oil (eugenol) in model crustaceans. Impluse, 1–21. https://impulse.appstate.edu/articles/2018/effects-clove-oil-eugenol-proprioceptive-neurons-heart-rate-and-behavior-model-crustac
Zakon HH (2012) Adaptive evolution of voltage-gated sodium channels: the first 800 million years. Proc Natl Acad Sci USA 109:10619–10625. https://doi.org/10.1073/pnas.1201884109
Zhu YC, Yocom E, Sifers J, Uradu H, Cooper RL (2016a) Modulatory effects on Drosophila larva hearts: room temperature, acute and chronic cold stress. J Comp Physiol A 186:829–841
Zhu YC, Uradu H, Majeed ZR, Cooper RL (2016b) Optogenetic stimulation of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol Rep 4(3):e12695. https://doi.org/10.14814/phy2.12695
Acknowledgements
Funded by Dept. of Biology, Univ. of KY. and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103436 (R.A.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stanley, C.E., Adams, R., Nadolski, J. et al. The effects of tricaine mesylate on arthropods: crayfish, crab and Drosophila. Invert Neurosci 20, 10 (2020). https://doi.org/10.1007/s10158-020-00243-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-020-00243-5