Abstract
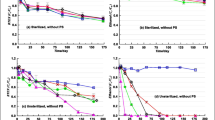
The combination of persulfate (PS) oxidation with enhanced bioremediation (EBR) is a potential trend in remediating organic-contaminated groundwater. However, the impacts of PS on EBR presented in the transition zone between PS oxidation zone and EBR zone need further study. To better characterize the impacts and provide available indicators, PS oxidation and EBR with nitrate amended were performed through the microcosm experiments to remove dissolved benzene, toluene, ethylbenzene and xylene (denoted as BTEX) in gasoline-saturated groundwater. The results indicated that PS oxidation combined with EBR almost completely removed BTEX with the ratio of > 93% over the experiments, which is better than PS oxidation (54–97%) but still worse than EBR (100%). The removal velocities of BTEX in EBR, PS oxidation, and PS oxidation combined with EBR were 0.94, 0.1–0.16, and 0.1–0.54 mg/L/d, respectively. High concentration of PS, along with high-strength activation, made the pH decrease to 3.3–4.4 and the Eh increase to 141–203 mV, thus greatly inhibited microbial activities as well. In such circumstances, oxygen and nitrate could not be significantly used as electron acceptors by microbials. To reduce the impacts of PS oxidation on EBR, the PS/BTEX molar ratio of < 6 and the PS/Fe2+ molar ratio of > 1 may be appropriate in transition zone. The hydro-chemical indicators, including pH, Eh, and availability of electron acceptors such as oxygen and nitrate, could reflect the impacts of PS oxidation on bioprocesses. During in-situ chemical oxidation (ISCO), PS injection and PS activation by Fe2+ should be managed for decreasing the impacts on EBR, based on the PS/BTEX and PS/Fe2+ molar ratios.







Similar content being viewed by others
References
Andrade LN, Araujo SF, Matos AT, Henriques AB, Oliveira LC, Souza PP, Chagas P, Leão MMD, Amorim CC (2017) Performance of different oxidants in the presence of oxisol: remediation of groundwater contaminated by gasoline/ethanol blend. Chem Eng J 308:428–437
Baciocchi R, D'Aprile L, Innocenti I, Massetti F, Verginelli I (2014) Development of technical guidelines for the application of in-situ chemical oxidation to groundwater remediation. J Clean Prod 77:47–55
Barker JF, Patrick GC, Major D (1987) Natural Attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monit Remote 7:64–71
Chen Y, Cheng Y, Jiang Y, Yan Y, Liu Z, Tong Q, Sun Z (2017) Pump-and-treat method to remove nitrate from groundwater with liquor as the carbon source. Environ Earth Sci 76:392. https://doi.org/10.1007/s12665-017-6729-z
Chen YD, Barker JF, Gui L (2008) A strategy for aromatic hydrocarbon bioremediation under anaerobic conditions and the impacts of ethanol: a microcosm study. J Contam Hydrol 96:17–31
Corseuil HX, Gomez DE, Schambeck CM, Ramos DT, Alvarez PJJ (2015) Nitrate addition to groundwater impacted by ethanol-blended fuel accelerates ethanol removal and mitigates the associated metabolic flux dilution and inhibition of BTEX biodegradation. J Contam Hydrol 174:1–9
Da Silva MLB, Corseuil HX (2012) Groundwater microbial analysis to assess enhanced BTEX biodegradation by nitrate injection at a gasohol-contaminated site. Int Biodeterior Biodegrad 67:21–27
Da Silva MLB, Ruiz-Aguilar MGL, Alvarez PJJ (2005) Enhanced anaerobic biodegradation of BTEX-ethanol mixtures in aquifer columns amended with sulfate, chelated ferric iron or nitrate. Biodegrad 16:105–114
Farhadian M, Duchez D, Vachelard C, Larroche C (2009) Accurate quantitative determination of monoaromatic compounds for the monitoring of bioremediation processes. Bioresour Technol 100:173–178
Liang C, Huang CF, Chen YJ (2008a) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100
Liang C, Huang CF, Mohanty N, Kurakalva RM (2008b) A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 73:1540–1543
Liu H, Bruton TA, Li W, Buren JV, Prasse C, Doyle FM, Sedlak DL (2016) Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(IV)-containing oxides: stoichiometric efficiency and transformation products. Environ Sci Technol 50:890–898
Liu H, Bruton TA, Doyle FM, Sedlak DL (2014) In situ chemical oxidation of contaminated groundwater by persulfate: decomposition by Fe(III)- and Mn(IV)-containing oxides and aquifer materials. Environ Sci Technol 48:10330–10336
Ma J, Yang Y, Jiang X, Xie Z, Li X, Chen C, Chen H (2018) Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 190:296–306
Mohseni-Bandpi A, Elliott DJ, Zazouli MA (2013) Biological nitrate removal processes from drinking water supply-a review. J Environ Health Sci Eng 11:35. https://doi.org/10.1186/2052-336X-11-35
Mora VC, Madueño L, Peluffo M, Rosso JA, Del Panno MT, Morelli IS (2014) Remediation of phenanthrene-contaminated soil by simultaneous persulfate chemical oxidation and biodegradation processes. Environ Sci Pollut Res 21:7548–7556
Nourmoradi H, Nikaeen M, Khiadani M (2012) Removal of benzene, toluene, ethylbenzene and xylene (BTEX) from aqueous solutions by montmorillonite modified with nonionic surfactant: equilibrium, kinetic and thermodynamic study. Chem Eng J 191:341–348
O'Connor D, Hou D, Ok YS, Song Y, Sarmah AK, Li X, Tack FMG (2018) Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: a review. J Controlled Release 283:200–213
Schreiber ME, Bahr JM (2002) Nitrate-enhanced bioremediation of BTEX-contaminated groundwater parameter estimation from natural-gradient tracer experiments. J Contam Hydrol 55:29–56
Shayan M, Thomson NR, Aravena R, Barker JF, Madsen EL, Marchesi M, DeRito C, Bouchard D, Buscheck TE, Kolhatkar R, Daniels EG (2018) Integrated plume treatment using persulfate coupled with microbial sulfate reduction. Ground Water Monit Remote 38:45–61
Sra KS, Thomson NR, Barker JF (2013) Persulfate injection into a gasoline source zone. J Contam Hydrol 150:35–44
Sutton NB, Grotenhuis JTC, Langenhoff AAM, Rijnaarts HHM (2011) Efforts to improve coupled in situ chemical oxidation with bioremediation: a review of optimization strategies. J Soil Sediment 11:129–140
Sutton NB, Kalisz M, Krupanek J, Marek J, Grotenhuis T, Smidt H, Weert J, Rijnaarts HHM, Gaans P, Keijzer T (2014) Geochemical and microbiological characteristics during in situ chemical oxidation and in situ bioremediation at a diesel contaminated site. Environ Sci Technol 48:2352–2360
Tsitonaki A, Petri B, Crimi M, Mosbaek H, Siegrist RL, Bjerg PL (2010) In situ chemical oxidation of contaminated soil and groundwater using persulfate: a review. Crit Rev Environ Sci Technol 40:55–91
Wiedemeier TH, Wilson JH, Kampbell DH (1999) Technical protocol for implementing intrinsic remediation with long-term monitoring for natural attenuation of fuel contamination dissolved in groundwater, vol 1. Air Force Center for Environmental Excellence, Technology Transfer Division Brooks Air Force Base, San Antonio, Texas
Zhao Y, Qu D, Hou Z, Zhou R (2015) Enhanced natural attenuation of BTEX in the nitrate-reducing environment by different electron acceptors. Environ Technol 36:615–621
Acknowledgments
The authors are grateful for the supports from the Project of National Natural Science Foundation of China (Grant Nos. 41967028) and key project of Guangxi Natural Science Foundation (Grant Nos. 2019GXNSFDA245030).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, Y., Cheng, Y., Li, L. et al. A microcosm study on persulfate oxidation combined with enhanced bioremediation to remove dissolved BTEX in gasoline-contaminated groundwater. Biodegradation 31, 213–222 (2020). https://doi.org/10.1007/s10532-020-09904-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-020-09904-z