Abstract
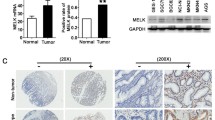
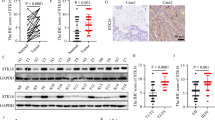
Maternal embryo leucine zipper kinase (MELK) has a higher expression level in a variety of cancers and involved in progression of colorectal cancer. The MELK expression levels in colorectal cancer tissues and cells were detected by RT-qPCR. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and transwell assays were used to examine the effect of the MELK konckdown on the proliferation, migration and invasion of colorectal cancer cells. Western blot analysis was used to detect the protein level of MELK and the downstream signaling pathways related proteins. Our findings indicated that MELK expression in colorectal cancer tissues was significantly higher than that in para-carcinoma tissues. Knockdown of MELK with shRNA had strong inhibition effects on the proliferation, migration and invasion of colorectal cancer cells. MELK knockdown could also decrease the phosphorylation level of AKT through FAK/Src pathway. Our results indicated downregulation of MELK retarded the progression of CRC by inhibition of the phosphorylation level of AKT through inactivating FAK/Src pathways. Therefore, MELK has the potential to be explored as a new therapeutic target and knockdown can be used as a potential treatment strategy for colorectal cancer.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Behjati S (2015) Colorectal cancer in miniature. Sci Transl Med 7(289):289ec85
Chen J, Elfiky A, Han M, Chen C, Saif MW (2014) The role of Src in colon cancer and its therapeutic implications. Clin Colorectal Cancer 13(1):5–13
Choi S, Ku JL (2011) Resistance of colorectal cancer cells to radiation and 5-FU is associated with MELK expression. Biochem Biophys Res Commun 412(2):207–213
Choi S-U, Kim KR, Cheon HZ, Shon Y (2015) Abstract B120: Maternal embryonic leucine zipper kinase (MELK) induces invasion and migration of glioblastoma. Exp Mol Ther 14(12 Supplement 2):B120
Chun-Feng Z, Liu SQ, Lin T, Qin MB, Liang MZ, Huang JA (2016) Effects of Sph K1 and FAK on epithelial-mesenchymal transition in colon cancer HCT116 cells. Chin J Pathophysiol 32(3):439–444
Dai C, Zhang X, Xie D, Tang P, Li C, Zuo Y et al (2017) Targeting PP2A activates AMPK signaling to inhibit colorectal cancer cells. Oncotarget 8(56):95810
Ganguly R, Mohyeldin A, Thiel J, Kornblum HI, Beullens M, Nakano I (2015) MELK—a conserved kinase: functions, signaling, cancer, and controversy. J Clin Transl Med 4(1):11
Goldstein DA, Zeichner SB, Bartnik CM, Neustadter E, Flowers CR (2016) Metastatic colorectal cancer: a systematic review of the value of current therapies. Clin Colorectal Cancer 15(1):1–6
Gu M-M, Gao D, Yao P-A, Yu L, Yang X-D, Xing C-G, Zhou J, Shang Z-F, Li M (2008) p53-inducible gene 3 promotes cell migration and invasion by activating the FAK/Src pathway in lung adenocarcinoma. Cancer Sci 109(12):3783–3793
Izzo JG, Dalby KN, Liang H, Yao H, Powis GJCR (2013) Abstract 2157: targeting the maternal embryonic leucin zipper kinase (MELK) in gastric cancers. Exp Mol Ther 73(8 Supplement):2157–2157
Knowles G, Haigh R, McLean C, Phillips HA, Dunlop MG, Din FV (2013) Long term effect of surgery and radiotherapy for colorectal cancer on defecatory function and quality of life. Eur J Oncol Nurs 17(5):570–577
Kohler RS, Kettelhack H, Knipprath-Mészaros AM, Fedier A, Schoetzau A, Jacob F, Heinzelmann-Schwarz V (2017) MELK expression in ovarian cancer correlates with poor outcome and its inhibition by OTSSP167 abrogates proliferation and viability of ovarian cancer cells. Gynecol Oncol 145(1):159–166
Kong L, Ding L (2016) Abstract 5166: gene expression microarray analysis identifies association of genes in the integrin/fak signaling pathway with claudin-7 in human colon cancer tissue. Tumor Biol 76(14 Supplement):5166–5166
Kuner R, Fälth M, Pressinotti NC, Brase JC, Puig SB, Metzger J (2013) The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J Mol Med 91(2):237–248
Lesko A, Ahlers C, Prosperi JR (2015) Abstract LB-066: adenomatous polyposis coli regulates epithelial morphogenesis and migration through FAK/Src signaling. Mol Cell Biol 75(15 Supplement):LB-066
Li S, Li Z, Guo T, Xing XF, Ji JFJO (2015) Maternal embryonic leucine zipper kinase serves as a poor prognosis marker and therapeutic target in gastric cancer. Oncotarget 7(5):6266–6280
Mahajan K, Mahajan NPJ (2012) PIK-independent AKT activation in cancers: a treasure trove for novel therapeutics. J Cell Physiol 227(9):3178–3184
Mcquade R, Stojanovska V, Bornstein J, Nurgali K (2017) Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem 24(15):1537–1557
Parizadeh SM, Parizadeh SA, Alizade-Noghani M, Jafarzadeh-Esfehani R, Ghandehari M, Mottaghi-Moghaddam A, Goldani F, Khazaei M, Ghayour-Mobarhan M, Ferns GA, Hassanian SM et al (2019) Association between non-alcoholic fatty liver disease and colorectal cancer. J Hepatol 13(7):633–641. https://doi.org/10.1080/17474124.2019.1617696
Pitner MK, Taliaferro JM, Dalby KN, Bartholomeusz C (2017) MELK: a potential novel therapeutic target for TNBC and other aggressive malignancies. Expert Opin Ther Targets 21(9):849–859
Qiang HE, Liang LJ, Peng BG, Shao-Qiang LI, Tang D, Ya L (2008) Oncogenic transformation, migration and invasiveness of cells induced by ErbB2 are mediated via FAK-Src-MAPK signaling pathway. Chin J Pathophysiol 24(12):2363–2365
Reiske HR, Zhao J, Han DC, Cooper LA, Guan JL (2000) Analysis of FAK-associated signaling pathways in the regulation of cell cycle progression. FEBS Lett 486(3):275–280
Ren A, Dong Y, Tsoi H, Yu J (2015) Detection of miRNA as non-invasive biomarkers of colorectal cancer. Int J Mol Sci 16(2):2810–2823. https://doi.org/10.3390/ijms16022810
Sloothaak DAM (2015) Evolving concepts in staging and treatment of colorectal cancer. Hepatology 3(4):369–382
Wang Y, Lee YM, Baitsch L, Huang A, Xiang Y, Tong H et al (2014) MELK is an oncogenic kinase essential for mitotic progression in basal-like breast cancer cells. Elife 3:e01763
Wu C, You J, Fu J, Wang X, Zhang Y (2016) Phosphatidylinositol 3-kinase/Akt mediates integrin signaling to control RNA polymerase I transcriptional activity. Mol Cell Biol 36(10):1555–1568
Yeh CM, Lin CW, Yang JS, Yang WE, Su SC, Yang SF (2016) Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget 7(16):21952
Funding
This work was supported by Science and Technology Fund Project of health and Family Planning Commission of Guizhou Province in 2018 (Grant Nos. gzwjkj-2018-1-075, gzwjkj-2018-1-035), Special project of academic new seedling cultivation and innovation exploration of Guizhou Medical University in 2018 (Grant No. [2018]5779-30) and State science and technology plan project of Qiandongnan Prefecture in 2019 (Grant No. Qiandongnan Kehe J Zi [2019] No. 145, Qiandongnan Kehe J Zi [2019] No. 146).
Author information
Authors and Affiliations
Contributions
RL and XL conceived and designed the experiments, GML and WZ analyzed and interpreted the results of the experiments, WG, FH and JJQ performed the experiments.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors state that there are no conflicts of interest to disclose.
Ethical Approval
This study was reviewed and approved by the Ethics Review Committee of Affiliated Hospital of Guizhou Medical University and was performed in accordance with Declaration of Helsinki. Every patient provided their informed consent in writing prior to their participation in the study. Animal protocols, housing, and care were performed with the approval of the Ethics Committee of Guizhou Medical University and conducted according to the guidelines set forth in the National Institutes of Health's (NIH) “Guide for the Care and Use of Laboratory Animals” (8th edition).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Zhan, W., Guo, W. et al. MELK Accelerates the Progression of Colorectal Cancer via Activating the FAK/Src Pathway. Biochem Genet 58, 771–782 (2020). https://doi.org/10.1007/s10528-020-09974-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-020-09974-x