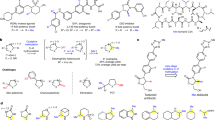
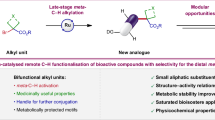
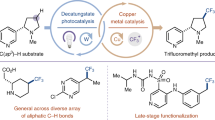
Abstract
The magic methyl effect is well acknowledged in medicinal chemistry, but despite its significance, accessing such analogues via derivatization at a late stage remains a pivotal challenge. In an effort to mitigate this major limitation, we here present a strategy for the cobalt-catalysed late-stage C–H methylation of structurally complex drug molecules. Enabling broad applicability, the transformation relies on a boron-based methyl source and takes advantage of inherently present functional groups to guide the C–H activation. The relative reactivity observed for distinct classes of functionalities were determined and the sensitivity of the transformation towards a panel of common functional motifs was tested under various reaction conditions. Without the need for prefunctionalization or postdeprotection, a diverse array of marketed drug molecules and natural products could be methylated in a predictable manner. Subsequent physicochemical and biological testing confirmed the magnitude with which this seemingly minor structural change can affect important drug properties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data, which include experimental procedures, references that support Fig. 1b, physicochemical, DMPK and pharmacological activity data for selected compounds, de novo synthesis strategies and NMR spectra, are available in the Supplementary Information.
References
Bergman, R. G. Organometallic chemistry: CH activation. Nature 446, 391–393 (2007).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, R. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013).
Larsen, M. A. & Hartwig, J. F. Iridium-catalyzed C–H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism. J. Am. Chem. Soc. 136, 4287–4299 (2014).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp 3)–H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Liao, K. et al. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 10, 1048–1055 (2018).
Willcox, D. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Barreiro, E. J., Kümmerle, A. E. & Fraga, C. A. M. The methylation effect in medicinal chemistry. Chem. Rev. 111, 5215–5246 (2011).
Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. Chem. Int. Ed. 52, 12256–12267 (2013).
Li, C., Liang, Y., Evans, R. W., Li, X. & MacMillan, D. W. C. Selective sp 3 C–H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017).
He, Z.-T., Li, H., Haydl, A. M., Whiteker, G. T. & Hartwig, J. F. Trimethylphosphate as a methylating agent for cross coupling: a slow-release mechanism for the methylation of arylboronic esters. J. Am. Chem. Soc. 140, 17197–17202 (2018).
Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp 2)–H and C(sp 3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Gandeepan, P. et al. 3d transition metals for C–H activation. Chem. Rev. 119, 2192–2452 (2019).
Evano, G. & Theunissen, C. Beyond Friedel and Crafts: directed alkylation of C–H bonds in arenes. Angew. Chem. Int. Ed. 58, 2–37 (2019).
Chen, Z. et al. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2, 1107–1295 (2015).
Graczyk, K., Haven, T. & Ackermann, L. Iron-catalyzed C(sp 2)–H and C(sp 3)–H methylations of amides and anilides. Chem. Eur. J. 21, 8812–8815 (2015).
Shang, R., Ilies, L. & Nakamura, E. Iron-catalyzed ortho C–H methylation of aromatics bearing a simple carbonyl group with methylaluminum and tridentate phosphine ligand. J. Am. Chem. Soc. 138, 10132–10135 (2016).
Thuy-Boun, P. S. et al. Ligand-accelerated ortho-C–H alkylation of arylcarboxylic acids using alkyl boron reagents. J. Am. Chem. Soc. 135, 17508–17513 (2013).
Neufeldt, S. R., Seigerman, C. K. & Sanford, M. S. Mild palladium-catalyzed C–H alkylation using potassium alkyltrifluoroborates in combination with MnF3. Org. Lett. 15, 2302–2305 (2013).
Rosen, B. R. et al. C–H functionalization logic enables synthesis of (+)-hongoquercin A and related compounds. Angew. Chem. Int. Ed. 52, 7317–7320 (2013).
Yoshino, T. & Matsunaga, S. (Pentamethylcyclopentadienyl)cobalt(iii)-catalyzed C–H bond functionalization: from discovery to unique reactivity and selectivity. Adv. Synth. Catal. 359, 1245–1262 (2017).
Moselage, M., Li, J. & Ackermann, L. Cobalt-catalyzed C–H activation. ACS Catal. 6, 498–525 (2016).
Weissman, S. A. & Anderson, N. G. Design of experiments (DoE) and process optimization. A review of recent publications. Org. Process Res. Dev. 19, 1605–1633 (2015).
Santanilla, A. B. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Tu, N. P. et al. High-throughput reaction screening with nanomoles of solid reagents coated on glass beads. Angew. Chem. Int. Ed. 58, 7987–7991 (2019).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–332 (2018).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalization chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Tomberg, A. et al. Relative strength of common directing groups in palladium-catalyzed aromatic C–H activation. iScience 20, 373–391 (2019).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Kutchukian, P. S. et al. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 7, 2604–2613 (2016).
Smith, J. M., Dixon, J. A., deGruyter, J. N. & Baran, P. S. Alkyl sulfinates: radical precursors enabling drug discovery. J. Med. Chem. 62, 2256–2264 (2019).
Uehling, M. R., King, R. P., Krska, S. W., Cernak, T. & Buchwald, S. L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 363, 405–408 (2019).
Dai, H.-X., Stepan, A. F., Plummer, M. S., Zhang, Y.-H. & Yu, J.-Q. Divergent C–H functionalizations directed by sulfonamide pharmacophores: late-stage diversification as a tool for drug discovery. J. Am. Chem. Soc. 133, 7222–7228 (2011).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Bäurle, S. et al. Identification of a benzimidazolecarboxylic acid derivative (BAY 1316957) as a potent and selective human prostaglandin E2 receptor subtype 4 (hEP4-R) antagonist for the treatment of endometriosis. J. Med. Chem. 62, 2541–2563 (2019).
Acknowledgements
The authors thank R. Sheppard and M. A. Hayes for valuable insight on biological testing, M. Härslätt and A. Ristinmaa for purification support, and M. A. Hayes and M. Lemurell for advice on the preparation of this manuscript. S.D.F. and M.J.J. acknowledge AstraZeneca and the AstraZeneca PostDoc program for their financial support. L.A. acknowledges the Georg-August-Universität Göttingen.
Author information
Authors and Affiliations
Contributions
S.D.F., M.J.J. and L.A. conceived the project and designed the experiments. M.J.J. and L.A. directed the project. S.D.F performed and analysed the experiments. S.D.F., M.J.J. and L.A. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental protocols and characterization for compounds mentioned in this work, references supporting Fig. 1b, overview of reaction optimization, description of competition experiments and compatibility screening, de novo synthesis strategies, PhysChem-, DMPK- and pharmacological activity data for selected compound and NMR spectra..
Rights and permissions
About this article
Cite this article
Friis, S.D., Johansson, M.J. & Ackermann, L. Cobalt-catalysed C–H methylation for late-stage drug diversification. Nat. Chem. 12, 511–519 (2020). https://doi.org/10.1038/s41557-020-0475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0475-7