Abstract
The effect of pulsed light (PL) on numerous important quality characteristics of pineapple juice was studied and compared with untreated and thermally pasteurised samples. The laboratory scale PL batch system used was operated with each three different voltages (1.8, 2.1, and 2.4 kV) and numbers of pulses (47, 94, and 187). Treatments with 2.4 kV and either 94 or 187 pulses (757/1479 J·cm−2) resulted in a 5-log reduction in aerobic mesophiles and the yeast and mould counts. Peroxidase was more resistant to PL than polyphenol oxidase, whereas the bromelain activity was completely retained in all PL-treated juices. Colour and antioxidant capacity were minimally affected, while vitamin C, genuine pineapple furanones, and phenolic compounds declined. In contrast, thermal pasteurisation was more detrimental to colour, antioxidant capacity, and vitamin C content, but resulted in a superior inactivation of microorganisms and enzymes and retention of phenolic compounds. Principal component analysis (PCA) permitted the differentiation of fresh, thermally pasteurised, and all PL-treated juices. PCA on the basis of the individual juice constituents additionally arranged the latter juices according to the number of pulses and voltage levels applied, particularly promoted by the oxidation of ascorbic to dehydroascorbic acid. In conclusion, PL treatment represents a promising new alternative to conventional thermal preservation techniques, whereby the inactivation of deteriorative enzymes may be further optimised.
Similar content being viewed by others
Introduction
Pineapples (Ananas comosus [L.] Merr.) are among the most popular tropical fruits. Consumers appreciate their fruity and exotic flavour. Moreover, they contain considerable amounts of vitamins, polyphenols, and other potentially health-promoting compounds (Steingass et al. 2015, 2016; Lobo and Paull 2017). In 2016, 26.2 million tons of pineapple have been produced worldwide. Based on the total world imports of 5.0 million tons, the estimated market share of fresh fruits accounted for 65%, whereas canned pineapples, pineapple concentrates, and single strength juices merely contributed to 20, 8, and 7%, respectively (FAO 2019).
Conventionally, pineapple juice is thermally pasteurised to attain a microbially safe product, i.e. a 5-log reduction in the most resistant pathogens (FDA 2004). Moreover, for an extended stability during refrigerated storage, the spoilage microbiota and enzymes in the juice need to be inactivated. Yeasts and moulds are the major spoilage-causing microorganisms in fruit juices (pH < 4.5). These are more resistant than pathogens, such as E. coli O157:H7, Salmonella spp., and Listeria monocytogenes, among others (Ağçam et al. 2018). Therefore, from an industrial perspective, a treatment ensuring the inactivation of both pathogenic and spoilage microorganisms is desirable. A wide range of different thermal pasteurisation conditions has been reported for pineapple juice. Among them, for instance, batch processing at 90 °C for either 1.5 min (Zheng and Lu 2011) or 5 min (Difonzo et al. 2019), and continuous treatment at 92–105 °C for 15–30 s (Hounhouigan et al. 2014).
Discolouration, alterations of the flavour, and the degradation of thermosensitive nutrients are major drawbacks of heat treatments, compromising the naturalness of the juices (Lobo and Paull 2017). In contrast, non-thermal processing methods may better retain the aforementioned quality attributes (Oms-Oliu et al. 2010). In particular, for the European market, there is a trend towards premium chilled juices with superior sensory quality, whereas the market share of conventionally pasteurised juices and those from concentrates significantly declines (AIJN 2018). Consequently, sophisticated non-thermal preservation technologies are highly demanded (Oms-Oliu et al. 2010; Koutchma 2009). High-pressure processing, pulsed electric fields, and UV treatment have been already proposed as alternative technologies for pineapple juice and nectar (Buzrul et al. 2008; Ferreira et al. 2009; Chia et al. 2012).
Pulsed light (PL) treatment is considered as another highly promising non-thermal technology. It employs a broad spectrum of high-intensity light (commonly ~100–1100 nm), covering ultraviolet (UV), visible (Vis), and near-infrared (NIR) wavelength ranges, applied in extremely short pulses. PL may find numerous applications in the food sector, not only for surface decontamination of solid foods and packaging materials but also for the treatment of liquids, such as beverages (Oms-Oliu et al. 2010). Unlike continuous UV light treatment, processing time for PL treatment with an equivalent lethality is significantly shorter, because the high-intensity polychromatic light pulses are instantly emitted from the xenon lamp without any pre-heating time. The efficacy of microbial inactivation by PL has been reported to result from photochemical, photothermal, and photophysical effects, minimally affecting nutritional and sensory attributes (Condón et al. 2014; Gómez-López et al. 2012). However, most of the previous studies focused on the inactivation of microorganisms and enzymes (Condón et al. 2014; Ferrario et al. 2014; Gómez-López et al. 2012; Oms-Oliu et al. 2010; Ferreira et al. 2009), whereas individual quality-determining constituents, such as vitamins, phenolic compounds, and carotenoids, have been largely disregarded. Over the past decade, the application of PL has been studied in several fruit juices, such as apple and orange juices (Palgan et al. 2011; Pataro et al. 2011). A few studies corroborated the application of continuous UV treatment on pineapple juice (Chia et al. 2012; Sew et al. 2014; Shamsudin et al. 2014), whereas its processing using PL has so far not been investigated.
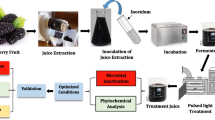
The present study aimed to explore the efficacy of PL on multiple important quality characteristics of pineapple juice, including microbial load, enzyme activities, colour, antioxidant capacity (AOC), and selected phytochemicals. In addition, aiming at the optimisation of the PL process, pineapple juices treated with various energy intensities were investigated and compared with fresh and thermally pasteurised samples.
Materials and Methods
Chemicals
All chemicals were acquired from VWR International (Leuven, Belgium and Fontenay-sous-Bois, France), Merck (Darmstadt, Germany), Sigma-Aldrich (Steinheim, Germany and Buchs, Switzerland), Carl Roth (Karlsruhe, Germany), Extrasynthèse (Genay, France), Th. Geyer (Renningen, Germany), or HiMedia Laboratories (Mumbai, India). Ultrapure and pure water was prepared with an arium 611 UV (Sartorius, Göttingen, Germany) ultrapure and an arium advance EDI type 2 (Sartorius India, Mumbai, India) pure water system, respectively, and used throughout, if not stated otherwise.
Pineapple Juice Production
Fully ripe, orange-yellow-fleshed pineapple fruits (Ananas comosus [L.] Merr.; ca. 1 kg per fruit) of the cv. ‘Queen’ were obtained from a local supplier near the Institute of Chemical Technology in Matunga, Mumbai, India.
Fresh Juice
After removing the crown, the surface of the pineapples was decontaminated by washing under running tap water followed by dipping into a 100 ppm hypochlorite solution for 1 min. The fruits were again thoroughly rinsed with pure water. Peel, eyes, and core were manually removed with a slicer. The remaining pulp was pre-cut into small cubes (1–2 cm3) and subsequently pureed in a household juicer (HL7701/00 Mixer Grinder, Philips India, Mumbai, India) at 6000 rpm for 5 min. The slurry obtained was filtered through a press-filter (Microfilt India, Mumbai, India) and passed through a stainless steel screen with a mesh size of 100 μm. The fresh pineapple juice was directly filled into polypropylene pouches (thickness 120 μm) and vacuum sealed or processed as follows.
The fresh pineapple juice had a total soluble solids content of 14.2 ± 0.4 °Brix (RHB-32ATC hand refractometer Erma 0-32 °B, Tokyo, Japan), a pH value of 4.0 ± 0.2 (CyberScan pH Tutor, Eutech Instruments, Singapore), and a titratable acidity (titration with 0.1 M NaOH to a pH value of 8.1) of 0.65 ± 0.01 g/100 g juice (expressed as citric acid).
Thermally Pasteurised Juice
For thermal pasteurisation, 50 mL of the fresh pineapple juice was transferred into polypropylene pouches, vacuum sealed, and placed in a water bath maintained at 90 ± 1 °C. The temperature at the core inside a dummy pouch was sensed by a K-type thermocouple (HI-93510, Hanna Equipment India, Mumbai, India). The treatment time of 5 min was started once the core reached the target temperature of 90 °C. The heat-up time lasted 190–195 s. The treatment time of 5 min at 90 °C was selected to target a 5-log reduction in resistant spoilage natural microbiota in the juice. Subsequently, the pouches were cooled in an ice bath (~2 °C).
PL-Treated Juices
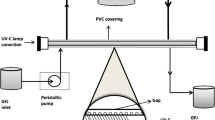
PL treatments were performed batch-wise in a benchtop laboratory scale high-intensity PL system (Xenon X-1100, Xenon, Wilmington, MA, USA) positioned in a laminar air flow chamber, as presented in Fig. 1. The laminar flow was maintained during decontamination of the cabinet and switched off prior to the PL treatments. The system consisted of a controller unit and a separate linear lamp housing (model LH-840) mounted on top of a sample tray made of glass. The controller unit contained an operator display, a capacitor, and a triggered transformer providing the required energy and initiating the pulses. The lamp housing was equipped with a linear xenon flash lamp (type B, Ø 2.54 × 40.6 cm), emitting high-intensity light in the wavelength range of 240–1100 nm, and a surrounding elliptical reflector (all from Xenon, Wilmington, MA, USA). Additionally, a blower was connected to the lamp housing providing forced-air cooling. A radiometer (model PE-50-DIF-C, Ophir Optronics Solutions, Jerusalem, Israel) was used to sense the fluence, i.e. the incident energy intensity (J·cm−2) on the juice surface. The initial juice temperature was 20 °C and merely rose by 1.7–7.2 °C during PL treatment (see Table 1) as monitored by using a K-type thermocouple (HI-93510, Hanna Equipment India, Mumbai, India).
Pineapple juices were treated in aliquots of 50 mL, filled into a glass cylinder (Ø 3.5 cm × 37.5 cm) with a longitudinal opening, resulting in a maximal layer thickness of 0.5 cm. The juice was exposed to PL in burst mode horizontally from the top with a perpendicular distance of 8.0 cm (x1 = 3.8 cm, x2 = 4.2 cm, see Fig. 1) between the front of the lamp and the top surface of the juice. Prior to treatment, the glass cylinder and the PL equipment surfaces were disinfected with aqueous ethanol (80%, v/v).
A 2-factor-3-level factorial design was employed for PL treatment investigating the effect of different voltage levels (1.8, 2.1, and 2.4 kV) and number of pulses (47, 94, and 187 pulses, applied during 30, 60, and 120 s, respectively). The pulse width of 360 μs was kept constant. The average fluences per pulse were 3.4, 5.4, and 8.0 J·cm−2 for 1.8, 2.1, and 2.4 kV, respectively (see Table 1). The corresponding frequency was 640 ms (1.56 Hz). After each treatment, the juice sample was immediately aseptically transferred into polypropylene pouches and cooled on ice.
For enzymatic, microbiological, and antioxidative analyses, all juice types were ice-cooled and immediately analysed. For the remaining analyses, the juice samples were frozen in dry ice, stored at − 35 °C as short as possible, and thawed in a 20 °C water bath shortly prior to use.
Microbial Counting
The serial dilution-pour plate technique was employed for microbial counting of aerobic mesophiles as well as yeasts and moulds according to Chakraborty et al. (2015b) by pouring 1 mL of juice or an appropriate dilution (made with sterile saline water) with the liquid agars into Petri dishes. For the determination of aerobic mesophiles as well as yeasts and moulds, the plates were incubated at 37 °C for 24 h and at 30 °C for 5 days, respectively. Microbial counts were expressed as colony-forming units (CFU) in log scale per millilitre of juice (log10 CFU/mL). Plates with <10 CFUs (<1 log10 CFU/mL) were not evaluated.
Enzyme Assays
Bromelain activity was determined by spectrophotometry according to the assay described by Jutamongkon and Charoenrein (2010) and expressed in casein digestive units (CDU). 1 CDU was defined by 1 μg of tyrosine released per minute per milligram of protein.
The activities of peroxidase (POD) and polyphenol oxidase (PPO) were quantitated according to the method described by Chakraborty et al. (2014). The activity unit (U) for POD or PPO represented the rate of change in product absorbance obtained per minute per milligram of protein in 1 mL juice.
All enzyme activities were finally expressed as residual activities (in %) based on the fresh, untreated juice.
Colour Profile
The colour of the pineapple juices was assessed in CIE L*a*b* colour space using a HunterLab colorimeter (LabScan XE, Hunter Associates Laboratory, VA, USA) with standard settings (D65/10°, reflection mode). Hue angle (h°), chromaticity (C*), and total colour difference (∆E*) were calculated from L* (lightness), a* (green-red), and b* (blue-yellow) values. In addition, the browning index as detailed in Chakraborty et al. (2015a) was calculated.
Antioxidant Capacity
The AOC was determined as reported by Chakraborty et al. (2015a) with slight modifications. In brief, 5 mL juice were combined with 15 mL aqueous methanol (80%, v/v), incubated for 2 h at 30 °C, and centrifuged (10,000 rpm equalling 6011×g, 10 min, 4 °C, CPR 30 Plus, Remi Laboratory Instruments, Mumbai, India). The supernatant was used for spectrophotometric AOC quantitation using the 2,2-diphenyl-1-picrylhydrazyl assay as described by Kaushik et al. (2014). AOC was expressed as milligram of gallic acid equivalents per 100 mL juice (mg GAE/100 mL).
Vitamin C Analysis
For the quantitation of vitamin C, i.e. ascorbic and dehydroascorbic acid (AA and DHAA), the sample preparation described by Aschoff et al. (2015) was adapted with slight modifications. Briefly, 0.5 mL pineapple juice was made up to a volume of 5 mL by adding aqueous 1.5% (w/v) meta-phosphoric acid solution with or without 20 mM tris(2-carboxyethyl)phosphine hydrochloride-reducing agent (pH 3.5, adjusted with 2 M K2HPO4). After mixing and incubation (30 min, where required), both solutions were membrane-filtered (0.45 μm, regenerated cellulose, Chromafil RC-45/15 MS, Macherey-Nagel, Düren, Germany) into amber glass vials.
Subsequent HPLC analyses were performed using an Hewlett Packard series 1100 HPLC system equipped with a G1315A diode array detector (all from Hewlett Packard, Waldbronn, Germany) as reported previously (Difonzo et al. 2019).
Analysis of Phenols, Furanones, Aromatic Amino Acids, and Amines
For HPLC analysis, pineapple juices were centrifuged (10,000×g, 10 min, miniSpin plus, Eppendorf, Hamburg, Germany) and the obtained supernatants membrane-filtered (0.45 μm, regenerated cellulose, Chromafil RC-45/15 MS, Macherey-Nagel, Düren, Germany) into amber glass vials.
For compound identification, HPLC-DAD-ESI-MSn analyses were conducted using an Agilent series 1100 HPLC system (Agilent Technologies, Waldbronn, Germany) coupled to an Esquire 3000+ ion-trap mass spectrometer with electrospray ionisation (ESI) source (Bruker Daltonics, Bremen, Germany) as reported by Steingass et al. (2015).
Quantitation was achieved applying an Acquity H-class UPLC system equipped with an eλ photodiode array detector (both from Waters, Milford, MA, USA). HPLC settings were as previously reported (Difonzo et al. 2019). Detection wavelengths were set to 260, 280, and 320 nm. UV/Vis spectra were recorded in the range of 200–700 nm. Quantitation was achieved using external calibrations of suitable reference standards as detailed elsewhere (Difonzo et al. 2019).
Carotenoid Analysis
Pineapple juice carotenoids were extracted under dimmed light, following a slightly modified procedure, according to Steingass et al. (2020). In brief, an aliquot of 2 mL pineapple juice was combined with 40 mg CaCO3, 20 mg NaHCO3, and 3 mL ice-cooled extraction solvent (methanol/ethyl acetate/light petroleum, 1:1:1, v/v/v). The latter contained each 0.1 g/L butylated hydroxyanisole and hydroxytoluene. The mixture was vigorously shaken for 10 s (Vortex Genie 2, Bender & Hobein, Zurich, Switzerland), followed by centrifugation (4500 rpm equalling 2173×g, 3 min, Heraeus Labofuge 200, Heraeus, Hanau, Germany). The upper organic phase was collected, and the aqueous phase was re-extracted with the aforementioned extraction solvent (3 × 2 mL). The pooled organic phase was finally washed with ice-cooled ultrapure water (2 × 3 mL) and subsequently evaporated to dryness at ambient temperature under a gentle stream of nitrogen. The carotenoids were dissolved in 200 μL methanol/tert-butyl methyl ether (1:1, v/v) assisted by ultrasonication, followed by membrane filtration (0.45 μm, polytetrafluoroethylene, Acrodisc CR 13, Pall, Ann Arbor, MI, USA) into amber glass vials, and immediately analysed by HPLC-DAD.
Carotenoids were quantitated using an Agilent series 1100 HPLC system equipped with a G1315B diode array detector (all from Agilent Technologies, Waldbronn, Germany). The column, eluents, and system settings were as detailed by Steingass et al. (2020). Injection volume was 20 μL. Carotenoids were monitored at 450 nm. UV/Vis spectra were recorded in the range of 200–800 nm.
All carotenoids were quantitated using a linear calibration curve of (all-E)-β-carotene and expressed as β-carotene equivalents in μg/100 mL juice. The concentration of the stock solution was determined by spectrophotometry (Britton 1995). Limit of detection (0.7 ng on column) and quantitation (1.0 ng) were estimated based on signal-to-noise ratios of 3:1 and 10:1, respectively. The extraction recovery for (all-E)-β-carotene was 92% (n = 3).
Statistical Analysis
All technological treatments were performed twice and analysed in duplicate. Results were reported as means ± standard deviations. Significant differences between means were tested at 95% confidence interval by one-way analysis of variance (ANOVA) and Tukey’s HSD test calculated with SAS software version 9.4 (SAS Institute, Cary, NC, USA), assuming normal distribution and homogeneity of variance.
Multivariate statistics were performed using Solo software release 8.2.1 (Eigenvector Research, Wenatchee, WA, USA). Prior to the calculation of the unsupervised principal component analysis (PCA), the ‘autoscale’ function of the software was used for initial data pre-processing, followed by the application of venetian blinds cross-validation.
Results and Discussion
Effect of PL on Microbial Counts
Figure 2 summarises the analysed microbial counts of all pineapple juices. In the fresh juice, the counts for aerobic mesophiles as well as yeasts and moulds were 5.8 and 6.0 log10 CFU/mL juice, respectively. The mildest treatment (1.8 kV/47 pulses, 160 J·cm−2) already caused a logarithmic reduction in aerobic mesophiles as well as yeasts and moulds by 2.0 and 2.2 log10 CFU/mL, respectively. As expected, at a fixed voltage level, lethality increased with increasing number of pulses. At 2.4 kV/94 pulses (757 J·cm−2), reduction in both aerobic mesophiles and yeast and mould counts was ~5 log cycles from the initial population. After both the most intense PL treatment (2.4 kV/187 pulses, 1479 J·cm−2) and thermal pasteurisation, microbial counts were below the detection limit.
Maximal log10 reductions of Saccharomyces cerevisiae in diverse fruit juices between 2 and 5 have been previously reported after UV-C or PL treatments (Paniagua-Martínez et al. 2018; Ferrario et al. 2014).
The UV-induced inactivation of natural microbiota in fruit juices has been well reported (Shamsudin et al. 2014; Chia et al. 2012; Noci et al. 2008). However, the mechanism of microbial inactivation by PL differs from that caused by continuous UV-C light (200–280 nm). The latter mainly inactivates microorganisms through photochemical effects, e.g. formation of thymine dimers, which thus inhibit DNA replication. PL is believed to affect microbial cells by two additional mechanisms: photothermal and photophysical effects (Gómez-López et al. 2012). Exposure to polychromatic light (200–1100 nm) in pulsation may generate localised temperature gradients within the microbial cells or at their surface, resulting in protein denaturation in the membranes. The photophysical effect causes changes in membrane permeability, shrinkage of the cytoplasmic membrane, and cell wall leakage, probably arising from short light bursts (Krishnamurthy et al. 2010). The higher extent of microbial inactivation after a higher number of pulses may be attributed to this photophysical, and also to photothermal and possibly synergistic effects (photochemical, photothermal, and photophysical) (Ferrario et al. 2014). The repeated pulsation for a longer period (up to 187 pulses) may have resulted in an immediate shock to the microbial cells, thus facilitating their internal collapse. However, a detailed study is required to comment on the antimicrobial efficacy of equivalent PL and continuous UV-treatments applied to pineapple juice.
Overall, our results clearly demonstrated, the higher the intensity of PL, the better the microbial inactivation. In addition, after an adequate number of pulses, photo-reactivation or damage-repair became less probable. Photoreactivation is the photolyase-catalysed breakdown of pyrimidine dimers, i.e. the photoproducts generated by UV exposure, during cell regeneration. The photolyase is activated during storage under light (Gómez-López et al. 2012). However, the extent of photo-reactivation has not been explored in the present study.
Effect of PL on Enzyme Activities
Bromelain is a mixture of several proteolytic enzymes with possible health-promoting effects (Maurer 2001). As illustrated in Fig. 3a, bromelain activity was heavily reduced by 93% after thermal pasteurisation, whereas PL did not affect its activity. Similar effects have been reported for pineapple juice after applying UV and/or mild heat treatments (Sew et al. 2014).
Activities of oxidoreductases in juices are one of the major causes of browning. The initial activities of POD and PPO in the fresh pineapple juice were 1673 and 617 U/mg, respectively. Both PL and thermal treatments significantly (p ≤ 0.05) reduced the enzyme activities to a varying extent (Fig. 3b and c). Thermal pasteurisation inactivated POD and PPO by ~81 and ~61%, respectively. PL was less effective in view of inactivation of these oxidoreductases. At the mildest PL treatment (1.8 kV/47 pulses, 160 J·cm−2), POD and PPO activities were diminished by 10 and 25%, respectively. The maximum inactivation of POD (− 42%) and PPO (− 50%) was observed after applying the strongest PL setting (2.4 kV/187 pulses, 1479 J·cm−2). However, with regard to colour stability, total inactivation of these oxidative enzymes in pure pineapple juices may be considered less important, since various inhibitors of enzymatic browning reactions, i.e. organic acids (AA, malic, and citric acid), l-cysteine, and pineapple-specific phenolic compounds, such as S-sinapyl-l-cysteine, N-l-γ-glutamyl-S-sinapyl-l-cysteine, and S-sinapyl glutathione (Wrolstad and Wen 2001; Chaisakdanugull et al. 2007), are present in the juice.
The data reported in the literature regarding the effect of UV light on oxidoreductases in fruit products are highly inconsistent. For instance, about 73% PPO inactivation was reported for clear apple juice exposed to UV-C light at 0.21 J·cm−2 for 95 min and 4 °C (Manzocco et al. 2009). On the other hand, Noci et al. (2008) did not observe significant reductions in PPO and POD activities in apple juice after being treated at 1.83 J·cm−2 UV-C for 30 min. Moreover, 39 and 84% inactivation of PPO activity in grape and apple juice were reported by Müller et al. (2014) after UV-C exposure for 25 cycles at 100 kJ·L−1 or 9.28 J·cm−2 in a coil reactor.
Inactivation of oxidoreductases upon UV-C light exposure has been attributed to photo-oxidation of proteins or their indirect oxidation due to the singlet oxygen species generated through a photosensitiser (Davies and Truscott 2001). The extent of enzyme inactivation was higher in the thermally pasteurised juice than in the PL-treated samples, possibly, due to different inactivation mechanisms. At elevated temperatures, e.g. 90 °C, heat-induced enzyme inactivation is believed to be caused by unfolding of a protein having higher activation energy along with loss or modification in few functional groups (Fortea et al. 2009). In contrast, even at the most severe PL treatment, the juice temperature did not increase beyond 7.2 °C (initial temperature 20 °C) in our experiments (see Table 1).
Effect of PL on Colour Profile
The fresh pineapple juice displayed CIE L*a*b* values of L* = 44.6, a* = − 2.3, and b* = 20.5 as well as a hue angle (h°) of 96.4°, a chromaticity (C*) of 20.6, and a browning index of 72.7 (see Table 2). Accordingly, the total colour difference (∆E*) of the fresh to all PL-treated juices varied only marginally between 0.8 and 1.3 and the treatment intensity did not cause significant differences, as illustrated in Fig. 4a. In contrast, the colour of the thermally pasteurised juice distinctly differed from that of the fresh juice (∆E* = 4.8). However, the browning indices were not significantly different for both thermally and PL-treated juices (see Table 2). The heat generated by PL (see Table 1) and thermal pasteurisation was probably low enough to avoid non-enzymatic browning. A ∆E* ≤ 3 has been reported to be not distinguishable by consumers (Patras et al. 2009). Similar findings have been reported for UV-treated pineapple juice, as the lightness was not influenced by UV irradiation (Chia et al. 2012; Shamsudin et al. 2014). On the other hand, Chia et al. (2012) have reported that thermally treated (80 °C/10 min) pineapple juices made of the cv. ‘Yankee’ had a darker appearance, as demonstrated by elevated b* values. This may be attributed to dark-coloured constituents formed during non-enzymatic Maillard browning during intense heating (Chia et al. 2012). However, in the present study, the differences in colour between fresh and thermally treated juices were mainly due to variations in L* and a* values (see Table 2). This may be attributed to the differing pineapple variety (here cv. ‘Queen’) and thermal processing conditions (90 °C/5 min).
Effect of PL on Antioxidant Capacity
The AOC of all pineapple juices is summarised in Fig. 4b. In the fresh juice, AOC was 16.4 ± 0.1 mg GAE/100 mL. PL did not significantly affect AOC, when 47 pulses, regardless of the voltage level, were applied (160–375 J·cm−2). However, AOC was significantly (p ≤ 0.05) reduced by an increased number of pulses (94 and 187; 325–1479 J·cm−2). The maximum reduction of AOC among all PL-treated juices was 14% at the most intense treatment (2.4 kV/187 pulses), though thermal pasteurisation resulted in an even higher loss of ~27%. The applied heat seemed to cause a significant irreversible damage to thermolabile antioxidants.
Literature data on the effect of PL on AOC in food products is limited. Palgan et al. (2011) observed a retention of AOC in apple juice treated with PL intensities of up to 14 J·cm−2, whereas a further increase up to 28 J·cm−2 significantly reduced AOC by ~6%. Moreira et al. (2015) observed insignificant differences in AOCs between untreated and PL-treated (12 J·cm−2) apple cubes. Particularly, regarding the effects of UV-treatment on AOC, inconsistent trends have been reported (Santhirasegaram et al. 2015; Sew et al. 2014) as, inter alia, the treatment intensity, the UV-dosage, and the product itself may affect the retention of antioxidants (Alothman et al. 2009).
Effect of PL on Phytochemical Composition
Vitamin C
Figure 5a displays the vitamin C concentrations, i.e. the sum of AA and DHAA, of all pineapple juices. In the fresh juice, vitamin C amounted to 18.8 ± 0.1 mg/100 mL. In contrast, the thermally pasteurised juice was characterised by a significant (p ≤ 0.05) drop to 7.7 ± 0.1 mg/100 mL, equalling a 59% reduction. This highlights the thermosensitive character of vitamin C. Declining vitamin C concentrations have been previously found in various fruit juices and beverages after thermal pasteurisation (Petruzzi et al. 2017; Goh et al. 2012). Considerably higher vitamin C concentrations in thermally pasteurised pineapple juices (also 90 °C/5 min, treated in glass bottles) produced from ‘MD2’ pineapples have been previously reported by Difonzo et al. (2019).
In case of the PL-treated pineapple juices, vitamin C was retained by mild PL settings, in particular, up to 2.1 kV/47 pulses. Subsequently, following higher energy intensities, the vitamin C concentrations declined to 13.4 ± 0.1 mg/100 mL (2.4 kV/187 pulses, 1479 J·cm−2, the most intensive PL treatment). This corresponds to a total vitamin C loss of 29%. The reduction of vitamin C tended to linearly follow the total fluence (R2 = 0.754, data not shown). Interestingly, Tran and Farid (2004) observed that the degree of vitamin C degradation was directly related to the UV-C dose (254 nm) applied (R2 = 0.975).
Noteworthy, in particular, the reduced form AA was negatively affected by PL. The degradation of AA was more pronounced with increasing voltage (1.8, 2.1, and 2.4 kV) and number of pulses (47, 94, and 187) applied. At each voltage level, a gradual degradation of AA was observed with increasing number of pulses. Concomitantly, the levels of DHAA partly rose, indicating that PL promoted the oxidation of AA to DHAA. A few studies have already demonstrated that specifically UV-C (254 nm) induced the photo-oxidation of AA in fruit juices and juice model systems. In addition, 2,3-diketogulonic acid has been reported as a further degradation product caused by UV-C (Tikekar et al. 2011a, b; Tran and Farid 2004). Tikekar et al. (2011a) proposed that the underlying mechanism is likely similar to the metal-catalysed oxidation of AA. Furthermore, in the presence of oxygen, oxidative enzymes, e.g. ascorbate oxidase and peroxidase (Davey et al. 2000), or local heat spots temporarily occurring at the surface (photothermal effect) without drastically increasing the overall sample temperature (Condón et al. 2014), may further promote vitamin C degradation.
Phenols, Furanones, Aromatic Amino Acids, and Amines
The total concentrations of phenols, furanones, aromatic amino acids, and amines in all pineapple juices are illustrated in Fig. 5b. The individual constituents are listed and numbered in Fig. 6, except for compound 9a/b/c, which was not quantitated due to the coelution of l-tryptophan, syringoyl hexoside, and p-coumaroyl aldarate (3). All compounds were identified on the basis of their retention times, UV/Vis spectra, and mass fragmentations, matching those reported and extensively discussed by Steingass et al. (2015) and Difonzo et al. (2019). The novel compound 15 was tentatively assigned to a sinapoyl dihexoside.
The prevailing phenolic compounds were N-l-γ-glutamyl-S-sinapyl-l-cysteine (18), S-sinapylglutathione (16), and S-sinapyl-l-cysteine (13), being consistent with previous reports (Difonzo et al. 2019; Steingass et al. 2017; Steingass et al. 2015; Wen and Wrolstad 2002; Wen et al. 1999). Particularly, such S-sinapyl derivatives were found to be efficient enzymatic browning inhibitors (Wrolstad and Wen 2001). Further phenolic compounds comprised diverse esters and glycosides of phenolic acids.
The thermally pasteurised juice displayed significantly (p ≤ 0.05) elevated concentrations of phenolic compounds, amounting to 39.8 mg/100 mL compared with ~21.7 and ~11.5–19.1 mg/100 mL in the fresh juice and the PL-treated samples, respectively. This may be attributed to the effective inactivation of both POD and PPO enzymes during thermal pasteurisation (see Fig. 3b, c), giving rise to a stabilised juice with regard to phenolic compounds.
In marked contrast to the thermal pasteurisation, PL technology had a significant (p ≤ 0.05) adverse effect on the total concentration of phenolic compounds. Depending on the PL settings, average losses ranged between 12 and 47%. Hereby, the effect was most detrimental for 187 pulses within each voltage level, each representing the highest total fluences (640, 1028, and 1479 J·cm−2). Decreasing concentrations of phenolic compounds have been previously reported for thermally pasteurised (90 °C) pineapple juice stored for up to 16 weeks under accelerated ageing conditions and light (320–800 nm) exposure (Steingass et al. 2017).
Considerably lower amounts of furanones in pineapple juices than those determined herein were reported by Difonzo et al. (2019). Thermal pasteurisation did not have an adverse effect on total furanones compared with the fresh juice. In contrast, mild PL settings (1.8 kV/47 pulses and 2.1 kV/47 pulses) seemed to promote a slight, however, insignificant rise in total furanones (on average up to 7%), followed by gradual losses of up to 35% (2.4 kV/187 pulses, 1479 J·cm−2). However, considering the aroma-active 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF), the fresh juice merely contained ~1.5 mg/100 mL, whereas its concentrations amounted to 4.8 mg/100 mL in the thermally pasteurised juice and ranged between 4.4 and 2.3 mg/100 mL in the PL-treated samples. HDMF is considered as a key odorant of fresh pineapples exerting “sweet, pineapple-like, caramel-like” odours and an odour threshold of 0.001 mg/100 mL in water; thus, it may also influence the odour of the pineapple juices assessed (Tokitomo et al. 2005).
The levels of aromatic amino acids and amines, comprising of l-tyrosine (1) and serotonin (2), were in the range of 11.5–15.3 mg/100 mL, and thus, higher than those reported by Wen and Wrolstad (2002) and Difonzo et al. (2019), which may be due to differing cultivars assessed. The effect of the individual treatments on the abovementioned compounds was negligible.
Carotenoids
Total carotenoids of all pineapple juices are illustrated in Fig. 5c, ranging between 183 and 240 μg/100 mL on average. The proportion of (all-E)-β-carotene accounted for 19–28%, corresponding to a concentration of 38–60 μg/100 mL. Carotenoids are sensitive to prolonged heat and light exposure (Britton and Khachik 2009). Surprisingly, the carotenoid concentrations in the fresh, the thermally pasteurised, and the PL-treated (160–1479 J·cm−2) juices were comparable. Contrasting results have been reported by Goh et al. (2012), although the total fluence of the UV-treatment of 7.5 mJ·cm−2 was far lower (unknown UV wavelength range). However, literature data regarding the effect of PL on carotenoids in juices is still limited.
The retinol activity equivalents calculated from (all-E)-β-carotene according to the recommendation by the Institute of Medicine (2001) ranged between 3.2 and 5.0 μg/100 mL on average among all juices assessed, thus being comparable with those previously reported for yellow-fleshed pineapples (Steingass et al. 2020).
The genuine carotenoid profile of pineapple pulp comprises (all-E)-violaxanthin, (all-E)-β-carotene, and diverse esters of (9Z)-violaxanthin (Steingass et al. 2020). During juice production, genuine fruit acids may trigger the acid-catalysed rearrangement of 5,6-epoxycarotenoids, such as violaxanthin, into corresponding furanoid 5,8-epoxycarotenoids, i.e. luteoxanthin, auroxanthin, and their stereoisomers (Britton et al. 1995). The latter are, in some instances, resolved on a C30 stationary phase. Consequently, numerous 5,8-epoxycarotenoids may be generated during pineapple juice production, as previously reported for orange juice made from concentrate (Meléndez-Martínez et al. 2008). This may explain that the previously reported violaxanthin isomers and their esters (Steingass et al. 2020) were only detected as minor constituents, whereas numerous additional carotenoid peaks at low abundance emerged in the chromatograms of our pineapple juices (not shown).
Pattern Recognition
Unsupervised PCA was used for pattern recognition among all analysed parameters and samples. The first data set subjected to PCA comprised microbial counts, enzyme activities, colour, and AOC. The second data set included the concentrations of the pineapple phytochemicals.
As illustrated in Fig. 6a, a', the first two principal components (PCs) explained 79% of the total variance (54% by PC1 and 25% by PC2) among the first data set. Fresh, thermally pasteurised, and PL-treated pineapple juices formed three entirely separated clusters. As deduced from the location of the loadings in Fig. 6a', in particular, the aerobic mesophilic count, yeast and mould count, and PPO activity contributed to the clear-cut differentiation of the fresh juice. The lightness and the total colour difference were the most decisive parameters promoting the distinct separation of the thermally pasteurised juice. In contrast, all PL-treated juices were heterogeneously clustered around the coordinate origin. Their clear-cut subdivision according to the individual PL settings applied was not observed.
Figure 6b, b' illustrates the scores and loadings of the second PCA. The first and the second PC explained 63 and 14%, respectively, corresponding to 77% of the total variance among the second data set. The thermally pasteurised samples formed one cluster that was clearly separated from the second one comprising the remaining juices. The position of the thermally pasteurised juice was highly related to the loadings of numerous phenolic compounds (13, 14, 17, and 18, among others, see Fig. 6b'), thus being in accordance to the elevated concentrations of phenolic compounds in these samples (see Fig. 5b). By contrast, the fresh juice and all PL-treated juices formed one S-like shaped cluster along on PC2. Consequently, the composition of the PL-treated juices resembled that of the fresh juice. Noteworthy, the fresh juice and the juices subjected to the mildest PL treatment (1.8 kV/47 pulses; 160 J·cm−2) were located at the top of the cluster, and thus, positively associated with AA. All juices treated with 187 pulses (1.8, 2.1, and 2.4 kV) were located in the lower part of the aforementioned cluster, and consequently, were highly related to DHAA. The remaining PL treatments were located in between and were partly mingled. In general, all PL settings were negatively related to almost all phenols, furanones, aromatic amino acids, and amines. However, the differences between the individual settings were small.
Conclusions
In conclusion, PL treatments higher than 757 J·cm−2 successfully reduced the microbial loads and were superior to thermal pasteurisation in preserving the contents of vitamin C, the AOC, the desired enzyme bromelain, and the colour. Thus, PL treatment is a promising new alternative to conventional thermal preservation techniques. Still, the inactivation of detrimental enzymes (POD and PPO) requires further optimisation. Consequently, PL treatment may be used to prolong the shelf life of, e.g. fresh-like chilled juices rather than producing shelf-stable pasteurised beverages.
Our study highlights PL as a viable technology for the non-thermal processing of pineapple juice at laboratory scale. However, successful upscaling will be required for its applicability in the food industry. In addition, future studies may focus on storage stability, guaranteeing shelf life extension and food safety, as well as aroma-determining volatiles and sensory analysis of PL-treated pineapple juices.
Abbreviations
- a * :
-
Green-red
- AA:
-
Ascorbic acid
- ANOVA:
-
Analysis of variance
- AOC:
-
Antioxidant capacity
- b * :
-
Blue-yellow
- C * :
-
Chromaticity
- CDU:
-
Casein digestive unit
- CFU:
-
Colony-forming unit
- ∆E* :
-
Total colour difference
- DHAA:
-
Dehydroascorbic acid
- ESI:
-
Electrospray ionisation
- GAE:
-
Gallic acid equivalent
- h° :
-
Hue angle
- HDMF:
-
4-Hydroxy-2,5-dimethyl-3(2H)-furanone
- HPLC-/UPLC-DAD:
-
High/ultra performance liquid chromatography-diode array detection
- L * :
-
Lightness
- MSn :
-
Multiple-stage mass spectrometry
- NIR:
-
Near-infrared
- PCA:
-
Principal component analysis
- PL:
-
Pulsed light
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- UV:
-
Ultraviolet
- Vis:
-
Visible
References
Ağçam, E., Akyıldız, A., & Dündar, B. (2018). Thermal pasteurization and microbial inactivation of fruit juices. In G. Rajauria & B. K. Tiwari (Eds.), Fruit juices: Composition, extraction, quality and analysis (pp. 309–339). Amsterdam: Elsevier.
AIJN (2018). Liquid fruit. Market report 2018. European Fruit Juice Association.
Alothman, M., Bhat, R., & Karim, A. A. (2009). Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends in Food Science & Technology, 20(5), 201–212. https://doi.org/10.1016/j.tifs.2009.02.003.
Aschoff, J. K., Kaufmann, S., Kalkan, O., Neidhart, S., Carle, R., & Schweiggert, R. M. (2015). In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. Journal of Agricultural and Food Chemistry, 63(2), 578–587. https://doi.org/10.1021/jf505297t.
Britton, G. (1995). UV/Visible spectroscopy. In G. Britton, S. Liaaen-Jensen, & H. Pfander (Eds.), Carotenoids: Volume 1b: Spectroscopy (pp. 13–62). Basel: Birkhäuser Verlag.
Britton, G., & Khachik, F. (2009). Carotenoids in food. In G. Britton, S. Liaaen-Jensen, & H. Pfander (Eds.), Carotenoids: Volume 5: Nutrition and health (pp. 45–66). Basel: Birkhäuser Verlag.
Britton, G., Liaaen-Jensen, S., & Pfander, H. (1995). Carotenoids: Volume 1a: Isolation and analysis. Basel: Birkhäuser Verlag.
Buzrul, S., Alpas, H., Largeteau, A., & Demazeau, G. (2008). Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. International Journal of Food Microbiology, 124(3), 275–278. https://doi.org/10.1016/j.ijfoodmicro.2008.03.015.
Chaisakdanugull, C., Theerakulkait, C., & Wrolstad, R. E. (2007). Pineapple juice and its fractions in enzymatic browning inhibition of banana [Musa (AAA group) Gros Michel]. Journal of Agricultural and Food Chemistry, 55(10), 4252–4257. https://doi.org/10.1021/jf0705724 .
Chakraborty, S., Rao, P. S., & Mishra, H. N. (2014). Effect of pH on enzyme inactivation kinetics in high-pressure processed pineapple (Ananas comosus L.) puree using response surface methodology. Food and Bioprocess Technology, 7(12), 3629–3645. https://doi.org/10.1007/s11947-014-1380-0.
Chakraborty, S., Rao, P. S., & Mishra, H. N. (2015a). Effect of combined high pressure–temperature treatments on color and nutritional quality attributes of pineapple (Ananas comosus L.) puree. Innovative Food Science and Emerging Technologies, 28, 10–21. https://doi.org/10.1016/j.ifset.2015.01.004.
Chakraborty, S., Rao, P. S., & Mishra, H. N. (2015b). Empirical model based on Weibull distribution describing the destruction kinetics of natural microbiota in pineapple (Ananas comosus L.) puree during high-pressure processing. International Journal of Food Microbiology, 211, 117–127. https://doi.org/10.1016/j.ijfoodmicro.2015.06.017 .
Chia, S. L., Rosnah, S., Noranizan, M. A., & Wan Ramli, W. D. (2012). The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. International Food Research Journal, 19(3), 1001–1010.
Condón, S., Álvarez, I., & Gayán, E. (2014). Non-thermal processing | Pulsed UV light. In Encyclopedia of Food Microbiology (pp. 974–981). Elsevier.
Davey, M. W., Van Montagu, M., Inzé, D., Sanmartin, M., Kanellis, A., Smirnoff, N., Benzie, I. J. J., Strain, J. J., Favell, D., & Fletcher, J. (2000). Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture, 80(7), 825–860. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6.
Davies, M. J., & Truscott, R. J. W. (2001). Photo-oxidation of proteins and its role in cataractogenesis. Journal of Photochemistry and Photobiology B: Biology, 63(1-3), 114–125. https://doi.org/10.1016/S1011-1344(01)00208-1 .
Difonzo, G., Vollmer, K., Caponio, F., Pasqualone, A., Carle, R., & Steingass, C. B. (2019). Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control, 96, 260–270. https://doi.org/10.1016/j.foodcont.2018.09.015 .
FAO (2019). FAOSTAT. Food and Agriculture Organization of the United States. http://www.fao.org/faostat/en/#data. Accessed 14 July 2019.
FDA (2004). Guidance for industry: Juice hazard analysis critical control point hazards and controls guidance. Center for Food Safety and Applied Nutrition, Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidanceindustry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first. Accessed 25 Oct 2019.
Ferrario, M., Guerrero, S., & Alzamora, S. M. (2014). Study of pulsed light-induced damage on Saccharomyces cerevisiae in apple juice by flow cytometry and transmission electron microscopy. Food and Bioprocess Technology, 7(4), 1001–1011. https://doi.org/10.1007/s11947-013-1121-9 .
Ferreira, E. H. d. R., Rosenthal, A., Calado, V., Saraiva, J., & Mendo, S. (2009). Byssochlamys nivea inactivation in pineapple juice and nectar using high pressure cycles. Journal of Food Engineering, 95(4), 664–669. https://doi.org/10.1016/j.jfoodeng.2009.06.053 .
Fortea, M. I., López-Miranda, S., Serrano-Martínez, A., Carreño, J., & Núñez-Delicado, E. (2009). Kinetic characterisation and thermal inactivation study of polyphenol oxidase and peroxidase from table grape (Crimson Seedless). Food Chemistry, 113(4), 1008–1014. https://doi.org/10.1016/j.foodchem.2008.08.053 .
Goh, S. G., Noranizan, M. A., Leong, C. M., Sew, C. C., & Sobhi, B. (2012). Effect of thermal and ultraviolet treatments on the stability of antioxidant compounds in single strength pineapple juice throughout refrigerated storage. International Food Research Journal, 19(3), 1131–1136.
Gómez-López, V. M., Koutchma, T., & Linden, K. (2012). Ultraviolet and pulsed light processing of fluid foods. In P. J. Cullen, B. K. Tiwari, & V. P. Valdramidis (Eds.), Novel thermal and non-thermal technologies (pp. 185–223). London: Elsevier.
Hounhouigan, M. H., Linnemann, A. R., Soumanou, M. M., & van Boekel, M. A. J. S. (2014). Effect of processing on the quality of pineapple juice. Food Reviews International, 30(2), 112–133. https://doi.org/10.1080/87559129.2014.883632 .
Institute of Medicine (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC.: National Academies Press.
Jutamongkon, R., & Charoenrein, S. (2010). Effect of temperature on the stability of fruit bromelain from Smooth Cayenne pineapple. Kasetsart Journal - Natural Science, 44, 943–948.
Kaushik, N., Kaur, B. P., Rao, P. S., & Mishra, H. N. (2014). Effect of high pressure processing on color, biochemical and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali). Innovative Food Science and Emerging Technologies, 22, 40–50. https://doi.org/10.1016/j.ifset.2013.12.011 .
Koutchma, T. (2009). Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food and Bioprocess Technology, 2(2), 138–155. https://doi.org/10.1007/s11947-008-0178-3 .
Krishnamurthy, K., Tewari, J. C., Irudayaraj, J., & Demirci, A. (2010). Microscopic and spectroscopic evaluation of inactivation of Staphylococcus aureus by pulsed UV light and infrared heating. Food and Bioprocess Technology, 3(1), 93–104. https://doi.org/10.1007/s11947-008-0084-8 .
Lobo, M. G., & Paull, R. E. (Eds.). (2017). Handbook of pineapple technology: Production, postharvest science, processing and nutrition. Chichester: John Wiley & Sons.
Manzocco, L., Quarta, B., & Dri, A. (2009). Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innovative Food Science and Emerging Technologies, 10(4), 506–511. https://doi.org/10.1016/j.ifset.2009.02.004 .
Maurer, H. R. (2001). Bromelain: Biochemistry, pharmacology and medical use. Cellular and Molecular Life Sciences, 58(9), 1234–1245. https://doi.org/10.1007/PL00000936.
Meléndez-Martínez, A. J., Britton, G., Vicario, I. M., & Heredia, F. J. (2008). The complex carotenoid pattern of orange juices from concentrate. Food Chemistry, 109(3), 546–553. https://doi.org/10.1016/j.foodchem.2008.01.003 .
Moreira, M. R., Tomadoni, B., Martín-Belloso, O., & Soliva-Fortuny, R. (2015). Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT - Food Science and Technology, 64(2), 1130–1137. https://doi.org/10.1016/j.lwt.2015.07.002 .
Müller, A., Noack, L., Greiner, R., Stahl, M. R., & Posten, C. (2014). Effect of UV-C and UV-B treatment on polyphenol oxidase activity and shelf life of apple and grape juices. Innovative Food Science and Emerging Technologies, 26, 498–504. https://doi.org/10.1016/j.ifset.2014.05.014 .
Noci, F., Riener, J., Walkling-Ribeiro, M., Cronin, D. A., Morgan, D. J., & Lyng, J. G. (2008). Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. Journal of Food Engineering, 85(1), 141–146. https://doi.org/10.1016/j.jfoodeng.2007.07.011 .
Oms-Oliu, G., Martín-Belloso, O., & Soliva-Fortuny, R. (2010). Pulsed light treatments for food preservation. A review. Food and Bioprocess Technology, 3(1), 13–23. https://doi.org/10.1007/s11947-008-0147-x.
Palgan, I., Caminiti, I. M., Muñoz, A., Noci, F., Whyte, P., Morgan, D. J., Cronin, D. A., & Lyng, J. G. (2011). Effectiveness of High Intensity Light Pulses (HILP) treatments for the control of Escherichia coli and Listeria innocua in apple juice, orange juice and milk. Food Microbiology, 28(1), 14–20. https://doi.org/10.1016/j.fm.2010.07.023.
Paniagua-Martínez, I., Ramírez-Martínez, A., Serment-Moreno, V., Rodrigues, S., & Ozuna, C. (2018). Non-thermal technologies as alternative methods for Saccharomyces cerevisiae inactivation in liquid media: A review. Food and Bioprocess Technology, 11(3), 487–510. https://doi.org/10.1007/s11947-018-2066-9.
Pataro, G., Muñoz, A., Palgan, I., Noci, F., Ferrari, G., & Lyng, J. G. (2011). Bacterial inactivation in fruit juices using a continuous flow Pulsed Light (PL) system. Food Research International, 44(6), 1642–1648. https://doi.org/10.1016/j.foodres.2011.04.048 .
Patras, A., Brunton, N., Da Pieve, S., Butler, F., & Downey, G. (2009). Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innovative Food Science and Emerging Technologies, 10(1), 16–22. https://doi.org/10.1016/j.ifset.2008.09.008 .
Petruzzi, L., Campaniello, D., Speranza, B., Corbo, M. R., Sinigaglia, M., & Bevilacqua, A. (2017). Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Comprehensive Reviews in Food Science and Food Safety, 16(4), 668–691. https://doi.org/10.1111/1541-4337.12270.
Santhirasegaram, V., Razali, Z., George, D. S., & Somasundram, C. (2015). Comparison of UV-C treatment and thermal pasteurization on quality of Chokanan mango (Mangifera indica L.) juice. Food and Bioproducts Processing, 94, 313–321. https://doi.org/10.1016/j.fbp.2014.03.011.
Sew, C. C., Mohd Ghazali, H., Martín-Belloso, O., & Noranizan, M. A. (2014). Effects of combining ultraviolet and mild heat treatments on enzymatic activities and total phenolic contents in pineapple juice. Innovative Food Science and Emerging Technologies, 26, 511–516. https://doi.org/10.1016/j.ifset.2014.05.008 .
Shamsudin, R., Noranizan, M. A., Pui Yee, Y., & Mansor, A. (2014). Effect of repetitive ultraviolet irradiation on the physico-chemical properties and microbial stability of pineapple juice. Innovative Food Science and Emerging Technologies, 23, 114–120. https://doi.org/10.1016/j.ifset.2014.02.005 .
Steingass, C. B., Glock, M. P., Schweiggert, R. M., & Carle, R. (2015). Studies into the phenolic patterns of different tissues of pineapple (Ananas comosus [L.] Merr.) infructescence by HPLC-DAD-ESI-MSn and GC-MS analysis. Analytical and Bioanalytical Chemistry, 407(21), 6463–6479. https://doi.org/10.1007/s00216-015-8811-2.
Steingass, C. B., Dell, C., Lieb, V. M., Mayer-Ullmann, B., Czerny, M., & Carle, R. (2016). Assignment of distinctive volatiles, descriptive sensory analysis and consumer preference of differently ripened and post-harvest handled pineapple (Ananas comosus [L.] Merr.) fruits. European Food Research and Technology, 242(1), 33–43. https://doi.org/10.1007/s00217-015-2515-x .
Steingass, C. B., Glock, M. P., Lieb, V. M., & Carle, R. (2017). Light-induced alterations of pineapple (Ananas comosus [L.] Merr.) juice volatiles during accelerated ageing and mass spectrometric studies into their precursors. Food Research International, 100, 366–374. https://doi.org/10.1016/j.foodres.2017.06.030.
Steingass, C. B., Vollmer, K., Lux, P. E., Dell, C., Carle, R., & Schweiggert, R. M. (2020). HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Research International, 127, 108709. https://doi.org/10.1016/j.foodres.2019.108709.
Tikekar, R. V., Anantheswaran, R. C., Elias, R. J., & LaBorde, L. F. (2011a). Ultraviolet-induced oxidation of ascorbic acid in a model juice system: Identification of degradation products. Journal of Agricultural and Food Chemistry, 59(15), 8244–8248. https://doi.org/10.1021/jf201000x.
Tikekar, R. V., Anantheswaran, R. C., & LaBorde, L. F. (2011b). Ascorbic acid degradation in a model apple juice system and in apple juice during ultraviolet processing and storage. Journal of Food Science, 76(2), H62–H71. https://doi.org/10.1111/j.1750-3841.2010.02015.x .
Tokitomo, Y., Steinhaus, M., Büttner, A., & Schieberle, P. (2005). Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Bioscience, Biotechnology, and Biochemistry, 69(7), 1323–1330. https://doi.org/10.1271/bbb.69.1323 .
Tran, M. T. T., & Farid, M. (2004). Ultraviolet treatment of orange juice. Innovative Food Science and Emerging Technologies, 5(4), 495–502. https://doi.org/10.1016/j.ifset.2004.08.002 .
Wen, L., & Wrolstad, R. E. (2002). Phenolic composition of authentic pineapple juice. Journal of Food Science, 67(1), 155–161. https://doi.org/10.1111/j.1365-2621.2002.tb11376.x .
Wen, L., Wrolstad, R. E., & Hsu, V. L. (1999). Characterization of sinapyl derivatives in pineapple (Ananas comosus [L.] Merill) juice. Journal of Agricultural and Food Chemistry, 47(3), 850–853. https://doi.org/10.1021/jf9808067.
Wrolstad, R. E., & Wen, L. (2001). Natural antibrowning and antioxidant compositions and methods for making the same (US6224926B1). United States Patent.
Zheng, H., & Lu, H. (2011). Use of kinetic, Weibull and PLSR models to predict the retention of ascorbic acid, total phenols and antioxidant activity during storage of pasteurized pineapple juice. LWT - Food Science and Technology, 44(5), 1273–1281. https://doi.org/10.1016/j.lwt.2010.12.023 .
Acknowledgements
Access to the facility of pulsed light treatment through Science & Engineering Research Board (SERB), Department of Science and Technology, India, and project fund (ECR/2016/001414) is gratefully acknowledged. Snehasis Chakraborty thanks the German Academic Exchange Service (DAAD) for the scholarship offered during the research. We also acknowledge Karin Scholten (University of Hohenheim) for her valuable support during carotenoid extraction.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vollmer, K., Chakraborty, S., Bhalerao, P.P. et al. Effect of Pulsed Light Treatment on Natural Microbiota, Enzyme Activity, and Phytochemical Composition of Pineapple (Ananas comosus [L.] Merr.) juice. Food Bioprocess Technol 13, 1095–1109 (2020). https://doi.org/10.1007/s11947-020-02460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02460-7