Abstract
Objective
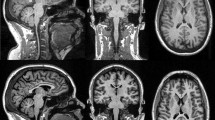
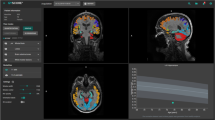
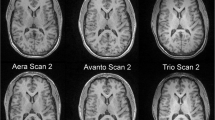
For clinical purposes and research projects in neurological disease, it is of interest to evaluate the performance and comparability of available sequences and software packages for brain volume assessment to determine whether they provide equivalent results. This study compares cross-sectional brain volume values derived from images obtained with MP-RAGE or MP2RAGE sequences, using SIENA/X, SPM, or MorphoBox.
Materials and methods
MP-RAGE and MP2RAGE T1-weighted images were obtained from 24 healthy volunteers. Back-to-back scans were performed in 12 of them. Brain volumes, coefficients of variation, and concordance coefficients were determined.
Results
Significant differences were found for most brain volumes derived from MP-RAGE and MP2RAGE images. MP2RAGE-derived measures showed a non-significant trend to larger coefficients of variation. There were statistical differences between brain volumes determined with the three software packages, whereas coefficients of variation were comparable for most brain volumes. Correlation and concordance values were lower for CSF and brain parenchyma fraction measures.
Conclusion
The results obtained advise caution when comparing brain volumes obtained by different sequences and software packages. Of note, for most brain volume measures, the MP2RAGE and MorphoBox coefficients of variation were similar to those obtained with MP-RAGE, SIENA/X or SPM, accepted tools for clinical research.





Similar content being viewed by others
References
Giorgio A, De Stefano N (2013) Clinical use of brain volumetry. J Magn Reson Imaging 37:1–14
Rocca MA, Battaglini M, Benedict RHB, De Stefano N, Geurts JJG, Henry RG et al (2017) Brain MRI atrophy quantification in MS from methods to clinical application. Neurology 88:403–413
Agosta F, Galantucci S, Filippi M (2017) Advanced magnetic resonance imaging of neurodegenerative diseases. Neurol Sci 38:41–51
De Guio F, Jouvent E, Biessels GJ, Black SE, Brayne C, Chen C et al (2016) Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab 36:1319–1337
Mugler JP III, Epstein FH, Brookeman JR (1992) Shaping the signal response during the approach to steady state in three-dimensional magnetization-prepared rapid gradient-echo imaging using variable flip angles. Magn Reson Med 28:165–185
Marques J, Kober T, Krueger G, van der Zwaag W, Van de Moortele P, Gruetter R (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49:1271–1281
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Schmitter D, Roche A, Maréchal B, Ribes D, Abdulkadir A, Bach-Cuadra M et al (2015) An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. Neuroimage Clin 7:7–17
ADNI MRI Scanner Protocols (2017) https://adni.loni.usc.edu/methods/documents/mri-protocols. Accessed 10 Sept 2019
Boto J, Gkinis G, Roche A, Kober T, Marechal B, Ortiz N et al (2017) Evaluating anorexia-related brain atrophy using MP2RAGE-based morphometry. Eur Radiol 27:5064–5072
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
O’Brien KR, Kober T, Hagmann P, Maeder P, Marques J, Lazeyras F et al (2014) Robust T1-weighted structural brain imaging and morphometry at 7T using MP2RAGE. PLoS ONE 9:e99676
Lin LI (1989) A concordance correlation-coefficient to evaluate reproducibility. Biometrics 45:255–268
McBride GB (2005) A proposal for strength-of-agreement criteria for Lin's concordance correlation coefficient. NIWA client report: HAM2005-062
Leigh R, Ostuni J, Pham D, Goldszal A, Lewis BK, Howard T et al (2002) Estimating cerebral atrophy in multiple sclerosis patients from various MR pulse sequences. Mult Scler 8:420–429
Horsfield MA, Rovaris M, Rocca MA, Rossi P, Benedict RHB, Filippi M et al (2003) Whole-brain atrophy in multiple sclerosis measured by two segmentation processes from various MRI sequences. J Neurol Sci 216:169–177
Chu RX, Tauhid S, Glanz BI, Healy BC, Kim G, Oommen VV et al (2016) Whole brain volume measured from 1.5T versus 3T MRI in healthy subjects and patients with multiple sclerosis. J Neuroimaging 26:62–67
Haller S, Falkovskiy P, Meuli R, Thiran J, Krueger G, Lovblad K et al (2016) Basic MR sequence parameters systematically bias automated brain volume estimation. Neuroradiology 58:1153–1160
Steenwijk MD, Amiri H, Schoonheim MM, De Sitter A, Barkhof F, Pouwels PJW et al (2017) Agreement of MSmetrix with established methods for measuring cross-sectional and longitudinal brain atrophy. Neuroimage Clin 15:843–853
Fujimoto K, Polimeni JR, van der Kouwe AJW, Reuter M, Kober T, Benner T et al (2014) Quantitative comparison of cortical surface reconstructions from MP2RAGE and multi-echo MPRAGE data at 3 and 7 T. Neuroimage 90:60–73
Seiger R, Hahn A, Hummer A, Kranz GS, Ganger S, Küblböck M et al (2015) Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3T MRI data. Neuroimage 113:207–216
Streitbuerger D, Pampel A, Krueger G, Lepsien J, Schroeter ML, Mueller K et al (2014) Impact of image acquisition on voxel-based-morphometry investigations of age-related structural brain changes. Neuroimage 87:170–182
Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A (2009) Evaluation of automated brain MR image segmentation and volumetry methods. Hum Brain Mapp 30:1310–1327
Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C (2012) Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS ONE 7:e45081
Tudorascu DL, Karim HT, Maronge JM, Alhilali L, Fakhran S, Aizenstein HJ et al (2016) Reproducibility and bias in healthy brain segmentation: comparison of two popular neuroimaging platforms. Front Neurosci 10:503
Wang C, Beadnall HN, Hatton SN, Bader G, Tomic D, Silva DG et al (2016) Automated brain volumetrics in multiple sclerosis: a step closer to clinical application. J Neurol Neurosurg Psychiatry 87:754–757
Duche Q, Raniga P, Egan GF, Acosta O, Gambarota G, Salvado O et al (2014) New partial volume estimation methods for MRI MP2RAGE. Med Image Comput Comput Assist Interv 17:129–136
Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L (1999) Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. multiple sclerosis collaborative research group. Neurology 53:1698–1704
Cardenas VA, Ezekiel F, Di Sclafani V, Gomberg B, Fein G (2001) Reliability of tissue volumes and their spatial distribution for segmented magnetic resonance images. Psychiatry Res Neuroimaging 106:193–205
Bermel RA, Sharma J, Tjoa CW, Puli S, Bakshi R (2003) A semiautomated measure of whole-brain atrophy in multiple sclerosis. J Neurol Sci 208:57–65
Lemieux L, Hammers A, Mackinnon T, Liu RSN (2003) Automatic segmentation of the brain and intracranial cerebrospinal fluid in T1-weighted volume MRI scans of the head, and its application to serial cerebral and intracranial volumetry. Magn Reson Med 49:872–884
Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL (2005) Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 64:1032–1039
Zivadinov R, Grop A, Sharma J, Bratina A, Tjoa CW, Dwyer M et al (2005) Reproducibility and accuracy of quantitative magnetic resonance imaging techniques of whole-brain atrophy measurement in multiple sclerosis. J Neuroimaging 15:27–36
Nakamura K, Fisher E (2009) Segmentation of brain magnetic resonance images for measurement of gray matter atrophy in multiple sclerosis patients. Neuroimage 44:769–776
de Boer R, Vrooman HA, Ikram MA, Vernooij MW, Breteler MMB, van der Lugt A et al (2010) Accuracy and reproducibility study of automatic MRI brain tissue segmentation methods. Neuroimage 51:1047–1056
Huppertz HJ, Kroll-Seger J, Kloppel S, Ganz RE, Kassubek J (2010) Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 49:2216–2224
Sampat MP, Healy BC, Meier DS, Dell'Oglio E, Liguori M, Guttmann CRG (2010) Disease modeling in multiple sclerosis: assessment and quantification of sources of variability in brain parenchymal fraction measurements. Neuroimage 52:1367–1373
Landman BA, Huang AJ, Gifford A, Vikram DS, Lim IAL, Farrell JAD et al (2011) Multi-parametric neuroimaging reproducibility: a 3-T resource study. Neuroimage 54:2854–2866
Acknowledgements
The authors thank Celine Cavallo for English language support.
Funding
This study was partially supported by La Fundació la Marató de TV3 and by Retos de Investigación DPI2017-86696-R (XLl).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JA, MA, and BM. The first draft of the manuscript was written by JA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Juli Alonso declares that he has no conflicts of interest. Deborah Pareto has received speaking honoraria from Novartis and Sanofi-Genzyme. Manel Alberich is sponsored by Novartis Farmacéutica SA Barcelona (Spain). Tobias Kober is an employee of Siemens Healthcare AG, Switzerland. Bénédicte Maréchal is an employee of Siemens Healthcare AG, Switzerland. Xavier Lladó declares that he has no conflicts of interest. Alex Rovira serves on scientific advisory boards for Novartis, Sanofi-Genzyme, Icometrix, and OLEA Medical, and has received speaker honoraria from Bayer, Sanofi-Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche, and Biogen Idec.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the hospital research and ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alonso, J., Pareto, D., Alberich, M. et al. Assessment of brain volumes obtained from MP-RAGE and MP2RAGE images, quantified using different segmentation methods. Magn Reson Mater Phy 33, 757–767 (2020). https://doi.org/10.1007/s10334-020-00854-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-020-00854-4