Chitosan Film Containing Mansoa hirsuta Fraction for Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Vegetal Material
2.3. Characterization of MHF UPLC-QTOF-MS/MS
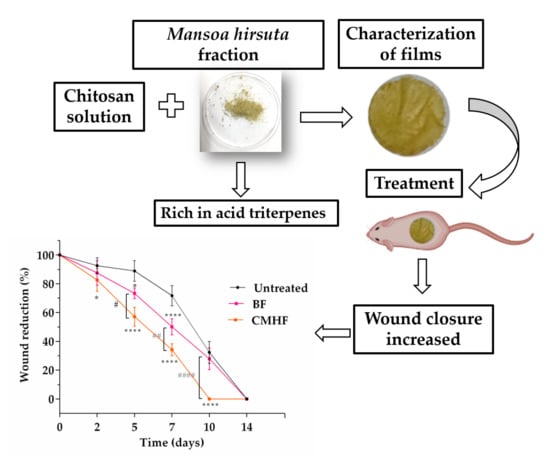
2.4. Preparation of Chitosan/M. hirsuta Fraction Films
2.5. Characterization of the Films
2.5.1. FTIR
2.5.2. XRD
2.5.3. TGA
2.5.4. DSC
2.5.5. SEM
2.5.6. AFM
2.5.7. Mechanical Properties
2.5.8. Films Thickness
2.6. In Vivo Wound Healing Study
2.6.1. Animals
2.6.2. Wound Healing Activity
Histological Analysis
Determination of Cytokine Concentration
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of MHF
3.2. Preparation and Characterization of the Films
3.2.1. Preparation of Chitosan/M. hirsuta Fraction Films
3.2.2. FTIR
3.2.3. XRD
3.2.4. Thermal Analysis
3.2.5. SEM and AFM
3.2.6. Mechanical Properties and Film Thickness
3.3. Wound Healing Activity
3.3.1. Histological Analysis
3.3.2. Cytokine Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evranos, B.; Aycan, D.; Alemdar, N. Production of ciprofloxacin loaded chitosan/gelatin/bone ash wound dressing with improved mechanical properties. Carbohydr. Polym. 2019, 222, 115007. [Google Scholar] [CrossRef]
- Venkataprasanna, K.S.; Prakash, J.; Vignesh, S.; Bharath, G.; Venkatesan, M.; Banat, F.; Sahabudeen, S.; Ramachandran, S.; Venkatasubbu, G.D. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020, 143, 744–762. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and Fabrication of Collagen-Coated Ostholamide Electrospun Nanofiber Scaffold for Wound Healing. ACS Appl. Mater. Interfaces. 2017, 9, 8556–8568. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.C.; Aldana, A.A.; Tártara, L.I.; Alovero, F.; Strumia, M.C.; Manzo, R.H.; Martinelli, M.; Jimenez-Kairuz, A.F. Bioadhesive and biocompatible films as wound dressing materials based on a novel dendronized chitosan loaded with ciprofloxacin. Carbohydr. Polym. 2017, 175, 75–86. [Google Scholar]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Di Filippo, M.F.; Panzavolta, S.; Albertini, B.; Bonvicini, F.; Gentilomi, G.A.; Orlacchio, R.; Passerini, N.; Bigi, A.; Dolci, L.S. Functional properties of chitosan films modified by snail mucus extract. Int. J. Biol. Macromol. 2020, 143, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Huang, Q.; Gha, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart Drug Delivery System Based on Biodegradable Chitosan / Poly (allylamine hydrochloride) Blend Films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Lopez-Galindo, A. Uses of clay minerals in semisolid health care and therapeutic products. Appl. Clay Sci. 2007, 36, 37–50. [Google Scholar] [CrossRef]
- Andreani, T.; Miziara, L.E.; Lorenzón, N.A.; De Souza, L.R.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremião, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Hirt-Burri, N.; Jeannerat, A.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Peptide-decorated chitosan derivatives enhance fibroblast adhesion and proliferation in wound healing. Carbohydr. Polym. 2016, 142, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Gómez, L.E.; Martel-Estrada, S.A.; Vargas-Requena, C.; Rivera-Armenta, J.L.; Alba-Baena, N.; Rodríguez-González, C.; Olivas-Armendáriz, I. Chitosan/Mimosa tenuiflora films as potential cellular patch for skin regeneration. Int. J. Biol. Macromol. 2016, 93, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.Y.; Jalil, J.; Ng, P.Y.; Ng, S.F. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Lemos, J.R.; Zappi, D.C. Distribuição geográfica mundial de plantas lenhosas da Estação Ecológica de Aiuaba, Ceará, Brasil. Rev. Bras. Biociências 2012, 10, 446–456. [Google Scholar]
- De Miranda Chaves, S.A.; Reinhard, K.J. Paleopharmacology and Pollen: Theory, Method, and Application. Mem. Inst. Oswaldo Cruz. 2003, 98, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Agra, M.D.F.; Silva, K.N.; Basílio, I.J.L.D.; De Freitas, P.F.; Barbosa-Filho, J.M. Survey of medicinal plants used in the region Northeast of Brazil. Braz. J. Pharmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Pereira, J.R.; Queiroz, R.F.; De Siqueira, E.A.; Brasileiro-Vidal, A.C.; Sant’Ana, A.E.G.; Silva, D.M.; De Mello Affonso, P.R.A. Evaluation of cytogenotoxicity, antioxidant and hypoglycemiant activities of isolate compounds from Mansoa hirsuta D.C. (Bignoniaceae). An. Acad. Bras. Cienc. 2017, 89, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Endringer, D.C.; Valadares, Y.M.; Campana, P.R.V.; Campos, J.J.; Guimarães, K.G.; Pezzuto, J.M.; Braga, F.C. Evaluation of Brazilian plants on cancer chemoprevention targets in vitro. Phyther. Res. 2010, 24, 928–933. [Google Scholar]
- Campana, P.R.V.; Mansur, D.S.; Gusman, G.S.; Ferreira, D.; Teixeira, M.M.; Braga, F.C. Anti-TNF-α activity of brazilian medicinal plants and compounds from Ouratea semiserrata. Phyther. Res. 2015, 10, 1509–1515. [Google Scholar] [CrossRef]
- Braga, F.C.; Wagner, H.; Lombardi, J.A.; De Oliveira, A.B. Screening the Brazilian flora for antihypertensive plant species for in vitro angiotensin-I-converting enzyme inhibiting activity. Phytomedicine. 2000, 7, 245–250. [Google Scholar] [CrossRef]
- Rocha, A.D.; De Oliveira, A.B.; De Souza Filho, J.D.; Lombardi, J.A.; Braga, F.C. Antifungal constituents of Clytostoma ramentaceum and Mansoa hirsuta. Phyther. Res. 2004, 18, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.D.M. Perfil Metabolômico e Farmacológico de Mansoa hirsuta D.C. (Bignoniaceae). Ph.D. Thesis, Universidade Federal de Alagoas, Maceió, Brazil, 6 August 2010. [Google Scholar]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Gramma, L.S.; Marques, F.M.; Vittorazzi, C.; de Andrade, T.A.M.; Frade, M.A.C.; de Andrade, T.U.; Endringer, D.C.; Scherer, R.; Fronza, M. Struthanthus vulgaris ointment prevents an over expression of inflammatory response and accelerates the cutaneous wound healing. J. Ethnopharmacol. 2016, 190, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Fabrication of asymmetric nanostarch reinforced Chitosan / PVP membrane and its evaluation as an antibacterial patch for in vivo wound healing application. Int. J. Biol. Macromol. 2018, 114, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.; Golden, B.; Sullivan, M.; Botros, S.M.; Miller, J.J.R.; Alshahrour, A.; Goldberg, R.P.; Sand, P.K. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007, 15, 80–86. [Google Scholar] [CrossRef]
- Novotny, L.; Abdel-Hamid, M.E.; Hamza, H.; Grancai, D. Development of LC- MS method for determination of ursolic acid: Application to the analysis of ursolic acid in Staphylea holocarpa Hemsl. J. Pharm. Biomed. Anal. 2003, 31, 961–968. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Fyhrquist, P.; Abdalla, A.M.A.; Abdelgadir, A.Y. LC-MS / MS Tandem Mass Spectrometry for Analysis of Phenolic Compounds and Pentacyclic Triterpenes in Antifungal Extracts of Terminalia brownii (Fresen). Antibiotics 2017, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-W.; Xing, Y.; Wen, C.; Yu, X.-X.; Sun, W.-L.; Xiu, Z.-L.; Dong, Y.-S. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorganic Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Rutkowska, E.; Szwerc, W.; Kocjan, R.; Latalski, M. Carlina species as a new source of bioactive pentacyclic triterpenes. Ind. Crops Prod. 2016, 94, 498–504. [Google Scholar] [CrossRef]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.R.; Brandner, J.; Schumann, H.; Bross, F.; Hoffmann, M.; Podmelle, F. Accelerating the aesthetic benefit of wound healing by triterpene. J. Cranio-Maxillofac. Surg. 2012, 40, e150–e154. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Neto, L.; Evaristo, F.F.V.; Alves, M.F.A.; Albuquerque, M.R.J.R.; Santos, H.S.; Bandeira, P.N.; Arruda, F.V.S.; Teixeira, E.H. Effect of the triterpene 3β, 6β, 16β-trihydroxylup-20(29)-ene isolated from the leaves of Combretum leprosum Mart. on cutaneous wounds in mice. J. Ethnopharmacol. 2015, 171, 116–120. [Google Scholar] [CrossRef]
- Sousa, G.F.; Duarte, L.P.; Alcântara, A.F.C.; Silva, G.D.F.; Vieira-Filho, S.A.; Silva, R.R.; Oliveira, D.M.; Takahashi, J.A. New triterpenes from Maytenus robusta: Structural elucidation based on NMR experimental data and theoretical calculations. Molecules 2012, 17, 13439–13456. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Mandal, A.; Rasul, M.G. A new bioactive ursane-type triterpenoid from Croton bonplandianum Bail. J. Chem. Sci. 2013, 125, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.Q.; Du, X.Y.; Huang, X.N.; Qiao, B. Enhanced oral bioavailability of ursolic acid nanoparticles via antisolvent precipitation with TPGS1000 as a stabilizer. J. Drug Deliv. Sci. Technol. 2015, 29, 210–217. [Google Scholar] [CrossRef]
- Antônio, E.; Antunes, O.R.; de Araújo, I.S.; Khalil, N.M.; Mainardes, R.M. Poly(lactic acid) nanoparticles loaded with ursolic acid: Characterization and in vitro evaluation of radical scavenging activity and cytotoxicity. Mater. Sci. Eng. C. 2017, 71, 156–166. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De La Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Abilova, G.K.; Kaldybekov, D.B.; Ozhmukhametova, E.K.; Saimova, A.Z.; Kazybayeva, D.S.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Chitosan/poly(2-ethyl-2-oxazoline) films for ocular drug delivery: Formulation, miscibility, in vitro and in vivo studies. Eur. Polym. J. 2019, 116, 311–320. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, P.K.; Sen, P. Preparation and characterization of optical property of crosslinkable film of chitosan with 2-thiophenecarboxaldehyde. Carbohydr. Polym. 2010, 80, 563–569. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch-chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Al-Nahary, T.T.; El-Ries, M.A.; Sultan, M.; Mabkhot, Y.N.; Al-Hussam, A.M. Thermal stability of anti-rheumatic pharmaceutical drugs parecoxib sodium and valdecoxib. J. Saudi Chem. Soc. 2012, 16, 177–182. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Beigi, A.A.M.; Heydari, R.; Khatibi, M. Thermal stability and decomposition kinetic studies of acyclovir and zidovudine drug compounds. Aaps Pharmscitech. 2013, 14, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparation and properties of carbohydrate-based composite films incorporated with CuO nanoparticles. Carbohydr. Polym. 2017, 169, 264–271. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Lu, Y.; Wu, T.; Pang, J.; Hu, Y. In situ self-assembly chitosan/ε-polylysine bionanocomposite film with enhanced antimicrobial properties for food packaging. Int. J. Biol. Macromol. 2019, 132, 385–392. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Pablo, J.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/ carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Švorcík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Sama, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, Z.; Zhang, Q.; Chen, F.; Yue, Z.; Zhang, T.; Deng, H.; Huselstein, C.; Anderson, D.P.; Chang, P.R.; et al. Accelerated skin wound healing by soy protein isolate–modified hydroxypropyl chitosan composite films. Int. J. Biol. Macromol. 2018, 118, 1293–1302. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, A.A.; Norziah, M.H. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Nam, G.W.; Kim, S.H.; Lee, S.H. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-α pathway. Exp. Dermatol. 2006, 15, 66–73. [Google Scholar] [CrossRef]
- Yang, X.; Yang, K.; Wu, S.; Chen, X.; Yu, F.; Li, J.; Ma, M.; Zhu, Z. Cytotoxicity and wound healing properties of PVA/ws-chitosan/glycerol hydrogels made by irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2010, 79, 606–611. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, S.P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Devalliere, J.; Dooley, K.; Yu, Y.; Kelangi, S.S.; Uygun, B.E.; Yarmush, M.L. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017, 141, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Mazumder, A.; Dwivedi, A.; Gerber, M.; du Plessis, J.; Hamman, J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017, 200, 1–7. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]











| Scores | Inflammatory Infiltrate | Neovascularization | Reepithelization | Granulation | Crust and Necrosis |
|---|---|---|---|---|---|
| 0 | Absent | Absent | Absent | Absent | Absent |
| 1 | Discrete | Initial | Partial | Present | Present |
| 2 | Moderate | Partial | Complete | - | - |
| 3 | Intense | Complete | - | - | - |
| Peak | tR (min) | [M-H]− | [M-H]− Calculated | [M-H]+ | [M-H]+ Calculated | MS2 Ions (ESI Positive) | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 2.57 | 487.3426 | 487.3428 | 489.3581 | 489.3574 | 471.3492 [M+H-H2O]+, 453.3381 [M+H-2H2O]+, 435.3277 [M+H-3H2O]+, 407.3332 [M+H-2H2O-HCOOH]+, 325.6983 [M+H-3H2O-HCOOH-4CH4]+ | Trihydroxyolean-en-oic acid or trihydroxyurs-en-oic acid |
| 2 | 3.09 | 487.3426 | 487.3428 | 489.3581 | 489.3574 | 471.3492 [M+H-H2O]+, 453.3381 [M+H-2H2O]+, 435.3277 [M+H-3H2O]+, 407.3332 [M+H-2H2O-HCOOH]+, 325.6983 [M+H-3H2O-HCOOH-4CH4]+ | Trihydroxyolean-en-oic acid or trihydroxyurs-en-oic acid (isomer) |
| 3 | 3.52 | 487.3426 | 487.3428 | 489.3577 | 489.3574 | 471.3492 [M+H-H2O]+, 453.3381 [M+H-2H2O]+, 435.3277 [M+H-3H2O]+, 407.3332 [M+H-2H2O-HCOOH]+, 325.6983 [M+H-3H2O-HCOOH-4CH4]+ | Trihydroxyolean-en-oic acid or trihydroxyurs-en-oic acid (isomer) |
| 4 | 3.72 | 485.3273 | 485.3272 | 487.3428 | 487.3418 | 469.3329 [M+H-H2O]+, 451.3232 [M+H-2H2O]+, 423.3286 [M+H-H2O-HCOOH]+, 405.3201 [M+H-2H2O-HCOOH]+, 324.6904 [M+H-3H2O-HCOOH-4CH4]+ | Trihydroxyurs-dien-oic acid or trihydroxyolean-dien-oic acid |
| 5 | 3.86 | 471.3479 | 471.3479 | 473.3634 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid |
| 6 | 4.25 | 471.3479 | 471.3479 | 473.3627 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid (isomer) |
| 7 | 4.36 | 471.3479 | 471.3479 | 473.3630 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid (isomer) |
| 8 | 4.52 | 469.3323 | 469.3323 | 471.3468 | 471.3468 | 453.3372 [M+H-H2O]+, 437.3422 [M+H-H2O-CH4]+, 425.2431 [M+H-HCOOH]+, 407.3314 [M+H-H2O-HCOOH]+, 325.6804 [M+H-2H2O-HCOOH-4CH4]+ | Hydroxy-oxoleana-en-oic acid or hydroxy-oxours-en-oic acid |
| 9 | 4.62 | 471.3479 | 471.3479 | 473.3629 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid (isomer) |
| 10 | 4.74 | 471.3479 | 471.3479 | 473.3631 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid (isomer) |
| 11 | 5.58 | 471.3479 | 471.3479 | 473.3636 | 473.3625 | 455.3531 [M+H-H2O]+, 437.3423 [M+H-2H2O]+, 409.3479 [M+H-H2O-HCOOH]+, 391.3400 [M+H-2H2O-HCOOH]+ | Dihydroxyurs-en-oic acid or dihydroxyolean-dien-oic acid (isomer) |
| 12 | 6.45 | 617.3858 | 617.3847 | 619.3985 | 619.3993 | 455.3524 [M+H-coumaroyl]+, 437.3419 [M+H-coumaroyl-H2O]+, 409.3480 [M+H-coumaroyl-HCOOH]+ | Coumaroyl-hydroxy-urs-en-oic acid |
| 13 | 6.95 | 455.3534 | 455.3530 | 457.3679 | 457.3673 | 439.3578 [M+H-H2O]+, 411.3625 [M+H-HCOOH]+, 393.3523 [M+H-HCOOH-H2O]+ | Ursolic acid or oleanolic acid |
| Samples | Wavenumber (cm−1) | Functional Groups and Types of Vibration | References |
|---|---|---|---|
| MHF | 3335 cm−1 | OH– stretching | [37] |
| 2928 cm−1 | CH3– (aliphatic) asymmetric stretching vibration | [36] | |
| 2864 cm−1 | CH3– (aliphatic) symmetric stretching | [36] | |
| 1688 cm−1 | C=O stretching vibration | [36] | |
| 1455 cm−1 | Angular deformation vibration of CH alkenes | [38] | |
| 1029 and 997 cm−1 | Symmetric C–O stretches and olefinic groups | [36] | |
| BF | 3351 cm−1 and 3274 cm−1 | OH– stretching which overlaps with NH–stretching | [40] |
| 2926 cm−1; 2866 cm−1 | CH2–; CH– stretching vibrations | [41] | |
| 1644 cm−1; 1547 cm−1 | C=O stretching (amide I); NH–bending (amide II) | [39] | |
| 1377 cm−1 | Acetamide groups | [39] | |
| 1150 cm−1 | Anti-symmetric stretching of the C–O–C bridge | [41] | |
| 1063 cm−1; 890 cm−1 | Skeletal vibrations involving the C–O stretching; vibration of C–C skeleton | [41] | |
| CMHF | 3274 cm−1 | NH–stretching | [40] |
| 2926 cm−1; 2866 cm−1 | CH2–; CH– stretching vibrations | [41] | |
| 1688 cm−1 | C=O stretching vibration | [36] | |
| 1455 cm−1 | Angular deformation vibration of CH alkenes | [38] | |
| 1372 cm−1 | Acetamide groups | [39] | |
| 1150 cm−1 | Anti-symmetric stretching of the C–O–C bridge | [41] | |
| 1075 cm−1 | Skeletal vibrations involving the C–O stretching; | [41] | |
| 1029 and 997 cm−1 | Symmetric C–O stretches and olefinic groups | [36] | |
| 890 cm−1 | Vibration of C–C skeleton | [41] |
| Samples | First Stage | Seconde Stage | Third Stage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Start (°C) | End (°C) | Wt. loss (%) | Start (°C) | End (°C) | Wt. Loss (%) | Start (°C) | End (°C) | Wt. Loss (%) | |
| MHF | 20 | 75 | 3.00 | 313 | 356 | 25.84 | 382 | 435 | 22.35 |
| BF | 44 | 109 | 23.88 | 191 | 262 | 19.41 | 277 | 370 | 11.90 |
| CMHF | 38 | 100 | 21.57 | 186 | 251 | 24.47 | 280 | 349 | 8.00 |
| Sample | Tensile Strength (Mpa) | Elongation at Break (%) | Thickness (μm) |
|---|---|---|---|
| BF | 9.19 ± 0.78 | 51.86 ± 10.80 | 57.89 ± 4.328 |
| CMHF | 22.60 ± 2.79 * | 68.75 ± 6.11 | 26.57 ± 2.052 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Pereira, J.; Suassuna Bezerra, G.; Alves Furtado, A.; de Carvalho, T.G.; Costa da Silva, V.; Lins Bispo Monteiro, A.; Bernardo Guerra, G.C.; de Araújo Júnior, R.F.; Sant’Ana, A.E.G.; Fernandes-Pedrosa, M.d.F.; et al. Chitosan Film Containing Mansoa hirsuta Fraction for Wound Healing. Pharmaceutics 2020, 12, 484. https://doi.org/10.3390/pharmaceutics12060484
Rodrigues Pereira J, Suassuna Bezerra G, Alves Furtado A, de Carvalho TG, Costa da Silva V, Lins Bispo Monteiro A, Bernardo Guerra GC, de Araújo Júnior RF, Sant’Ana AEG, Fernandes-Pedrosa MdF, et al. Chitosan Film Containing Mansoa hirsuta Fraction for Wound Healing. Pharmaceutics. 2020; 12(6):484. https://doi.org/10.3390/pharmaceutics12060484
Chicago/Turabian StyleRodrigues Pereira, Joquebede, Gabriela Suassuna Bezerra, Allanny Alves Furtado, Thaís Gomes de Carvalho, Valéria Costa da Silva, Amanda Lins Bispo Monteiro, Gerlane Coelho Bernardo Guerra, Raimundo Fernandes de Araújo Júnior, Antônio Euzébio Goulart Sant’Ana, Matheus de Freitas Fernandes-Pedrosa, and et al. 2020. "Chitosan Film Containing Mansoa hirsuta Fraction for Wound Healing" Pharmaceutics 12, no. 6: 484. https://doi.org/10.3390/pharmaceutics12060484