Abstract
Bacterial blight in pomegranate caused by Xanthomonas axonopodis pv. punicae (Xap) is an increasing threat to pomegranate cultivation in India. To prevent economic losses, it is pivotal to detect the infection in latent stages rather than in symptomatic stages. We have developed an enhanced loop-mediated isothermal amplification (LAMP) technique for the detection of latent Xap infection in pomegranate using six sets of pathovar-specific primers. The DNA-intercalating dye ethidium bromide was standardized for visualizing positive amplification in the LAMP assay. The optimum reaction time was 30 min or more at 65 °C, and assay sensitivity was enhanced down to femtogram (fg) amounts of template DNA (0.153 fg) in the LAMP assay. In artificially inoculated plants, the enhanced LAMP assay detected Xap on the 7th day post inoculation (dpi), while PCR detected Xap only after the 11th dpi. Finally, the specificity of the LAMP assay for Xap was validated against 31 bacterial strains belonging to 22 species. The LAMP assay developed in this study is highly specific, sensitive and robust and can be used as an improved alternative for PCR-based methods for the early detection of Xap infection in pomegranate. Early detection of Xap in pomegranate ensures quality fruits with increased yield by adopting precautionary measures that ensure improved monetary benefit to farmers.

Similar content being viewed by others
Change history
06 June 2020
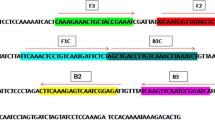
The images and caption for Fig. 1 is incorrect. Please view the correct Fig. 1 here.
References
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods :541–555
Bhadra S, Jiang YS, Kumar MR, Johnson RF, Hensley LE, Ellington AD (2015) Real-time sequence-validated loop-mediated isothermal amplification assays for detection of Middle East respiratory syndrome coronavirus (MERS-CoV). PLoS One 10(4):e0123126
Chan LL, Lo SCL, Hodgkiss IJ (2002) Proteomic study of a model causative agent of harmful red tide, Prorocentrum triestinum I: Optimization of sample preparation methodologies for analyzing with two-dimensional electrophoresis. Proteomics :1169–1186
Chandra R, Babu K, Jadhav V, Teixeira da Silva J (2010) Origin, history and domestication of pomegranate. Veg Cereal Sci Biotechnol 4:1–6
Cheng HR, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett 28:55–59
Chol BK, Wyss C, Göbel UB (1996) Phylogenetic analysis of pathogen-related oral spirochetes. J Clin Microbiol 34:1922–1925
Fagan PK, Hornitzky MA, Bettelheim KA, Djordjevic SP (1999) Detection of shiga-like toxin (stx1 and stx2), intimin (eaeA), and enterohemorrhagic Escherichia coli (EHEC) hemolysin (EHEC hlyA) genes in animal feces by multiplex PCR. Appl Environ Microbiol 65:868–872
Gilligan C (2008) Sustainable agriculture and plant diseases: an epidemiological perspective. Philos Trans R Soc Lond B Biol Sci 363:741–759
Hoopes LLM (2012) Nucleic acid blotting: Southern and northern. Curr Protoc Essent Lab Tech 2012:et0802s6
Jalikop SH (2007) Linked dominant alleles or inter-locus interaction results in a major shift in pomegranate fruit acidity of “Ganesh”דKabul Yellow”. Euphytica 158:201–207
Janda JM, Abbott SL (2007) 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45(9):2761–2764
Jun-hai N, Yue-rong G, Jun-mei Y, Qing-yun L, Guang-sui Y, Cun W et al (2015) Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of bacterial blight pathogen (Xanthomonas axonopodis pv. dieffenbachiae) in anthurium. Eur J Plant Pathol 142:801–813
Khater M, de la Escosura-Muñiz A, Merkoçi A (2017) Biosensors for plant pathogen detection. Biosens Bioelectron 93:72–86
Kubota R, Vine BG, Alvarez AM, Jenkins DM (2008) Detection of Ralstonia solanacearum by loop-mediated isothermal amplification. Phytopathol 98:1045–1051
Kumari P, Singh R, Punia R (2017) Evaluation of different inoculation methods for mango anthracnose disease development. Int J Curr Microbiol App Sci 6:3028–3032
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159
Leclerc M, Doré T, Gilligan CA, Lucas P, Filipe JAN (2014) Estimating the delay between host infection and disease (incubation period) and assessing its significance to the epidemiology of plant diseases. PLoS One 9:e0086568
López MM, Bertolini E, Olmos A, Caruso P, Gorris MT, Llop P, et al (2003) Innovative tools for detection of plant pathogenic viruses and bacteria. Int Microbiol :233–243
Lorenz TC (2012) Polymerase chain reaction: Basic protocol plus troubleshooting and optimization strategies. J Vis Exp. https://doi.org/10.3791/3998
Mandal PK, Biswas AK, Choi K, Pal UK (2011) Methods for rapid detection of food borne pathogens: An overview. Am J Food Technol 1:87–102
Mondal KK, Rajendran TP, Phaneendra C, Mani C, Sharma J, Shukla R, et al (2012) The reliable and rapid polymerase chain reaction (PCR) diagnosis for Xanthomonas axonopodis pv. punicae in pomegranate. Afr J Microbiol Res 6:5950–5956
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289:150–154
Munson MA, Banerjee A, Watson TF, Wade WG (2004) Molecular analysis of the microflora associated with dental caries. J Clin Microbiol 42:3023–3029
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Obura E, Masiga D, Wachira F, Gurja B, Khan ZR (2011) Detection of phytoplasma by loop-mediated isothermal amplification of DNA (LAMP). J Microbiol Methods 84:312–316
Pu J, Xie Y, Zhang H, Zhang X, Qi Y, Peng J (2014) Development of a real-time fluorescence loop-mediated isothermal amplification assay for rapid and quantitative detection of Fusarium mangiferae associated with mango malformation. Physiol Mol Plant Pathol 86:81–88
Qiao YM, Guo YC, Zhang XE, Zhou YF, Zhang ZP, Wei HP et al (2007) Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett 29:1939–1946
Rekha V, Rana R, Remesh AT, Bharathan AP, Kalluvila JJ, Gopinath D et al (2014) LAMP– a novel nucleic acid based diagnostic assay. Adv Anim Vet Sci 2:344–350
Sharma J, Sharma KK, Kumar A, Mondal KK, Thalor S, Maity A, Gharate R, Chinchure S, Jadhav VT (2017) Pomegranate bacterial blight: Symptomatology and rapid inoculation technique for Xanthomonas axonopodis pv. punicae. J Plant Pathol 99(1):109–119
Sheet OH, Grabowski NT, Klein G, Abdulmawjood A (2016) Development and validation of a loop mediated isothermal amplification (LAMP) assay for the detection of Staphylococcus aureus in bovine mastitis milk samples. Mol Cell Probes 30:320–325
Suwancharoen D, Kulchim C, Chirathaworn C, Yoshida S (2012) Development of a novel primer combination to detect pathogenic Leptospira by loop-mediated isothermal amplification. J Microbiol Methods 91:171–173
Van de Peer Y, Chapelle S, De Wachter R (1996) A quantitative map of nucleotide substitution rates in bacterial rRNA. Nucleic Acids Res 24:3381–3391
Wu GP, Chen SH, Levin RE (2015) Application of ethidium bromide monoazide for quantification of viable and dead cells of Salmonella enterica by real-time loop-mediated isothermal amplification. J Microbiol Methods 117:41–48
Acknowledgements
Our team would like to thank A. Satish (Dept. of Soil Science, UASB) for assisting in GIS mapping construction. We are grateful to Basavaraj (Dept. of Plant Pathology, UASB), Shankarappa (Dept. of Horticulture, Bagalkot), and Vinay Kumar (Dept. of Soil Science, UASB) for their technical assistance. The authors are highly thankful to Dr. Rashmi Aggarwal, Head and Principal Scientist, Dr. K. K. Mondal Principal Scientist, Division of Plant Pathology, ICAR-IARI New Delhi, India, for providing a bacterial culture (Xanthomonas campestris pv. campestris) and Edwin Raj (Plant Physiology and Biotechnology, UPASI Tea Research Institute) for his support in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1882 kb)
Rights and permissions
About this article
Cite this article
Prasannakumar, M.K., Buela Parivallal, P., Manjunatha, C. et al. Loop-mediated isothermal amplification assay for pre-symptomatic stage detection of Xanthomonas axonopodis pv. punicae infection in pomegranate. Australasian Plant Pathol. 49, 467–473 (2020). https://doi.org/10.1007/s13313-020-00720-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00720-w