Abstract
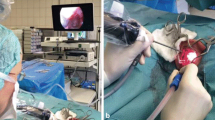
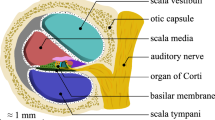
The aim of this work is to design, fabricate and experimentally validate a miniature steerable digital endoscope that can provide comprehensive, high-resolution imaging of the middle ear using a trans-nasal approach. The motivation for this work comes from the high incidence of middle ear diseases, and the current reliance on invasive surgery to diagnose and survey these diseases which typically consists of the eardrum being lifted surgically to directly visualize the middle ear using a trans-canal approach. To enable less-invasive diagnosis and surveillance of middle ear disease, we propose an endoscope that is small enough to pass into the middle ear through the Eustachian tube, with a steerable tip that carries a 1 Megapixel image sensor and fiber-optic illumination to provide high-resolution visualization of critical middle ear structures. The proposed endoscope would enable physicians to diagnose middle ear disease using a non-surgical trans-nasal approach instead, enabling such procedures to be performed in an office setting and greatly reducing invasiveness for the patient. In this work, the computational design of the steerable tip based on computed tomography models of real human middle ear anatomy is presented, and these results informed the fabrication of a clinical-scale steerable endoscope prototype. The prototype was used in a pilot study in three cadaveric temporal bone specimens, where high-quality middle ear visualization was achieved as determined by an unbiased cohort of otolaryngologists. This is the first paper to demonstrate cadaveric validation of a digital, steerable, clinical-scale endoscope for middle ear disease diagnosis, and the experimental results illustrate that the endoscope enables the visualization of critical middle ear structures (such as the epitympanum or sinus tympani) that were seldom or never visualized in prior published trans-Eustachian tube endoscopy feasibility studies.











Similar content being viewed by others
References
Ayache, D., V. Darrouzet, F. Dubrulle, C. Vincent, S. Bobin, M. Williams, and C. Martin. Imaging of non-operated cholesteatoma: clinical practice guidelines. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 129:148–152, 2012.
Barath, K., A. Huber, P. Stampfli, Z. Varga, and S. Kollias. Neuroradiology of cholesteatomas. Am. J. Neuroradiol. 32:221–229, 2011.
Bruns, T. L., K. E. Riojas, D. S. Ropella, M. S. Cavilla, A. J. Petruska, M. H. Freeman, R. F. Labadie, J. J. Abbott, and R. J. Webster. Magnetically steered robotic insertion of cochlear-implant electrode arrays: system integration and first-in-cadaver results. IEEE Robot. Automat. Lett. 5:2240–2247, 2020.
Caversaccio, M., W. Wimmer, J. Anso, G. Mantokoudis, N. Gerber, C. Rathgeb, D. Schneider, J. Hermann, F. Wagner, O. Scheidegger, M. Huth, L. Anschuetz, M. Kompis, T. Williamson, B. Bell, K. Gavaghan, and S. Weber. Robotic middle ear access for cochlear implantation: first in man. PLOS ONE August:1–12, 2019.
Chang, P. and S. Kim. Cholesteatoma: diagnosing the unsafe ear. Austral. Fam. Phys. 37:631–640, 2008.
Crowson, M. G., V. H. Ramprasad, N. Chapurin, C. D. Cunningham, and D. M. Kaylie. Cost analysis and outcomes of a second-look tympanoplasty-mastoidectomy strategy for cholesteatoma. Laryngoscope 126:2574–2579, 2016.
Dahroug, B., B. Tamadazte, S. Weber, L. Tavernier, and N. Andreff. Review on otological robotic systems: toward microrobot-assisted cholesteatoma surgery. IEEE Rev. Biomed. Eng. 11:125–142, 2018.
Di Martino, E., L. E. Walther, and M. Westhofen. Endoscopic examination of the eustachian tube: a step-by-step approach. Otol. Neurol. 26:1112–1117, 2005.
Dillon, N. P., R. Balachandran, J. M. Fitzpatrick, M. A. Siebold, R. F. Labadie, G. B. Wanna, T. J. Withrow, and I. Webster, Robert J. A compact, bone-attached robot for mastoidectomy. J. Med. Dev. 9, 031003, 2015.
DTIC. MIL-STD-1472D: Section on Rotary Controls , 1989.
Fedorov, A., R. Beichel, J. Kalpathy-Cramer, J. Finet, J.-C. Fillion-Robin, S. Pujol, C. Bauer, D. Jennings, F. Fennessy, M. Sonka, J. Buatti, S. Aylward, J. V. Miller, S. Pieper, and R. Kikinis. 3d slicer as an image computing platform for the quantitative imaging network. Magnet. Resona. Imaging 30:1323–1341, 2012.
Fichera, L., N. P. Dillon, D. Zhang, I. S. Godage, M. A. Siebold, B. I. Hartley, J. H. Noble, P. T. Russell, R. F. Labadie, and R. J. Webster. Through the eustachian tube and beyond: a new miniature robotic endoscope to see into the middle ear. IEEE Robot. Automat. Lett. 2:1488–1494, 2017.
Gulati, M., S. Gupta, A. Prakash, A. Garg, and R. Dixit. HRCT imaging of acquired cholesteatoma: a pictorial review. Insights Imaging 10:4–11, 2019.
Haginomori, S.-I., A. Takamaki, R. Nonaka, and H. Takenaka. Residual cholesteatoma: incidence and location in canal wall down tympanoplasty with soft-wall reconstruction. Arch. Otolaryngol. 134:652–657, 2008.
Hannula, S., R. Bloigu, K. Majamaa, M. Sorri, and E. Mäki-Torkko. Ear diseases and other risk factors for hearing impairment among adults: an epidemiological study. Int. J. Audiol. 51:833–840, 2012.
Heywood, R. L. and A. A. Narula. The pros and cons of canal wall up versus canal wall down mastoidectomy for cholesteatoma. Otorhinolaryngologist 6:140–143, 2013.
Ho, S. and J. Kveton. Efficacy of the 2-staged procedure in the management of cholesteatoma. Arch. Otolaryngol. 129:541–545, 2003.
Jaiprakash, A., W. B. O’Callaghan, S. L. Whitehouse, A. Pandey, L. Wu, J. Roberts, and R. W. Crawford. Orthopaedic surgeon attitudes towards current limitations and the potential for robotic and technological innovation in arthroscopic surgery. J. Orthopaed. Surg. 25:1–6, 2017.
Karhuketo, T. S. and H. J. Puhakka. Middle ear imaging via the eustachian tube with a superfine fiberoptic videomicroendoscope. ORL 60:30–34, 1998.
Keeler, J. A. and D. M. Kaylie. Cholesteatoma: is a second stage necessary? Laryngoscope 126:1499–1500, 2016.
Klug, C., B. Fabinyi, and M. Tschabitscher. Endoscopy of the middle ear through the eustachian tube: anatomic possibilities and limitations. Am. J. Otol. 20:209–303, 1999.
Kuo, C.-L., A.-S. Shiao, M. Yung, M. Sakagami, H. Sudhoff, C.-H. Wang, C.-H. Hsu, and C.-F. Lien. Updates and knowledge gaps in cholesteatoma research. BioMed Res. Int. 2015:1–16, 2015.
LaValle, S. M. Planning Algorithms. Cambridge: Cambridge University Press, 2006.
Lingam, R. K., R. Nash, A. Majithia, A. Kalan, and A. Singh. Non-echoplanar diffusion weighted imaging in the detection of post-operative middle ear cholesteatoma : navigating beyond the pitfalls to find the pearl. Insights Imaging 7:669–678, 2016.
Linstrom, C. J., C. A. Silverman, A. Rosen, and L. Z. Meiteles. Eustachian tube endoscopy in patients with chronic ear disease. Laryngoscope 110:1884–1889, 2000.
Louw, L. Acquired cholesteatoma pathogenesis: stepwise explanations. J. Laryngol. Otol. 124:587–593, 2010.
Miroir, M., Y. Nguyen, O. Sterkers, and A. B. Grayeli. Design, kinematic optimization, and evaluation of a teleoperated system for middle ear microsurgery. Sci. World J. 2012:1–19, 2012.
Möller, T. and B. Trumbore. Fast, minimum storage ray-triangle intersection. J. Graph. Tools 2:21–28, 1997.
Noble, J. H., B. M. Dawant, F. M. Warren, and R. F. Labadie. Automatic identification and 3D rendering of temporal bone anatomy. Otol. Neurotol. 30:436–442, 2009.
Paltura, C., T. S. Can, B. K. Yilmaz, M. E. Dinç, Ö. N. Develiolu, and M. Külekçi. Eustachian tube diameter: is it associated with chronic otitis media development? Am. J. Otolaryngol. 38:414–416, 2017.
Plouin-Gaudon, I., D. Bossard, S. Ayari-Khalfallah, and P. Froehlich. Fusion of MRIs and CT scans for surgical treatment of cholesteatoma of the middle ear in children. Arch. Otolaryngol. 136:878–883, 2010.
Poe, D., E. Rebeiz, M. Pankratov, and S. Shapshay. Transtympanic Endoscopy of the Middle Ear. The Laryngoscope 102:993–996, 1992.
Pusalkar, A. G. Cholesteatoma and Its Management. Indian Journal of Otolaryngology and Head & Neck Surgery 67:201–204, 2015.
Swaney, P. J., P. A. York, H. B. Gilbert, J. Burgner-Kahrs, and R. J. Webster. Design, fabrication, and testing of a needle-sized wrist for surgical instruments. Journal of medical devices 11:014501, 2017.
Swearingen, B. Update on Pituitary Surgery. The Journal of Clinical Endocrinology & Metabolism 97:1073–1081, 2012.
Todt, I., R. Seidl, A. Ernst, I. Todt, R. Seidl, and A. Ernst. A new minimally invasive method for the transtubal, microendoscopic application of fluids to the middle ear application of fluids to the middle ear. Minimally Invasive Therapy and Allied Technologies 17:300–302, 2009.
York, P. A., P. J. Swaney, H. B. Gilbert, and R. J. Webster. A wrist for needle-sized surgical robots. In: 2015 IEEE International Conference on Robotics and Automation (ICRA), pp. 1776–1781. 2015.
Zhang, D., M. L. Bennett, R. F. Labadie, and J. H. Noble. Simulation of trans-nasal endoscopy of the middle ear for visualization of cholesteatoma. In: 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), pp. 1415–1418, IEEE2015.
Acknowledgments
The authors would like to acknowledge Enable, Inc. for assisting in the design, development, and fabrication of the steerable endoscope presented in this work.
Funding
The authors thank the National Institutes of Health (NIH) for Grant R21 DC016153-01A1 which supported the work described in this paper. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Institutes of Health. Role of Sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Conflict of interest
The authors have no conflicts of interest to report.
Ethical Approval
All temporal bone experiments were performed under the supervision of a single otolaryngologist (M.F.). All cadaver specimens were obtained from Vanderbilt University Medical Center, and their use falls under IRB Exemption according to 45 CFR 46. We did not recruit a multiple-user cohort to perform these pilot experiments. Recruitment of subjects for the purposes of Likert survey data collection was performed under the approved IRB protocol 200449. The computer simulations run on patient models were deemed exempt from the Worcester Polytechnic Institute IRB pursuant to 45 CFR 46.101(b). Based on these consideration, and the fact that no live patients or live animals were involved in our experiments, prior approval from an ethics committee (IRB or IACUC) was not required.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ka-Wai Kwok oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gafford, J., Freeman, M., Fichera, L. et al. Eyes in Ears: A Miniature Steerable Digital Endoscope for Trans-Nasal Diagnosis of Middle Ear Disease. Ann Biomed Eng 49, 219–232 (2021). https://doi.org/10.1007/s10439-020-02518-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02518-9