Glycera sheikhmujibi n. sp. (Annelida: Polychaeta: Glyceridae): A New Species of Glyceridae from the Saltmarsh of Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sample Collection and Analysis
3. Results
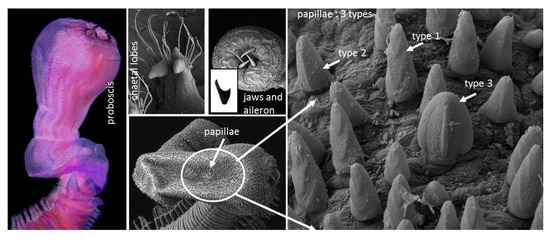
3.1. Systematics
3.2. Material Examined
3.3. Generic Identification
3.4. Diagnosis
3.5. Description
3.5.1. Holotype
3.5.2. Etymology
3.6. Distribution, Ecology, and Habitat
4. Discussion
- Proboscideal papillae do not have a terminal fingernail structure 2– Proboscideal papillae have a terminal fingernail structure 6
- There is one postchaetal lobe in all parapodia 3– There are two postchaetal lobes (at least) on the mid-body parapodia 5
- Mid-body, the notopodial prechaetal lobes are shorter than the neuropodial lobes, and branchiae are absentGlycera lapidum Quatrefages, 1866 [24].– Mid-body, the prechaetal lobes are about same length or longer than the notopodial lobes. Branchiae are present or absent 4
- The proboscideal papillae are digitiform and without ridges, ailerons have deeply incised bases, and simple digitiform branchiae are situated termino-dorsally on the parapodiaGlycera sphyrabrancha Schmarda, 1861 [12].– Conical proboscideal papillae with 5–20 transverse ridges, ailerons have slightly arched bases, and branchiae are absentGlycera oxycephala Ehlers, 1887 [25].
- Ailerons have gently incised bases; long, mid-body postchaetal lobes are digitiform and of about equal length; three types of proboscideal papillae, with the main type having fewer than three ridges; and branchiae are absent Glycera sheikhmujibi n. sp.– Ailerons have an interamal plate and triangular bases; mid-body parapodia have slender, triangular, notopodial and distinctly shorter, rounded neuropodial postchaetal lobes. The retractile branchiae are situated medially on anterior side of parapodia 7
- Digitiform proboscideal papillae have a straight, median, longitudinal ridgeGlycera tesselata Grube, 1863 [26].– Digitiform proboscideal papillae have 6–20 transverse ridgesGlycera brevicirris Grube, 1870 [13].
- Parapodia have slender, triangular, notopodial and distinctly shorter, rounded, neuropodial postchaetal lobes; and branchiae are simple, digitiform, and retractileGlycera nicobarica Grube, 1867 [18].– Parapodia have two slender, triangular postchaetal lobes of about the same length, or notopodial lobes that are only slightly longer than the neuropodial lobes; branchiae are digitiform and retractile with 1–2 rami Glycera unicornis Lamarck, 1818 [14].
- The mid-body parapodia have two slender. triangular postchaetal lobes of about the same length 9– The mid-body parapodia have slender, triangular notopodial and shorter, more or less rounded, neuropodial postchaetal lobes 10
- Parapodia do not have branchiae Glycera onomichiensis Izuka, 1912 [27].– There are 1–5 digitiform branchial rami situated dorsally on the parapodial basesGlycera cinnamomea Grube, 1874 [19].
- In mid-body and posterior parapodia, the neuropodial postchaetal lobes are more or less rounded. Simple digitiform branchiae are situated termino-dorsally on the parapodia 11– In the posterior parapodia are neuropodial postchaetal lobes as long as the notopodial lobes, and equally slender and triangular. Simple digitiform branchiae are situated medio-dorsally on the parapodia Glycera posterobranchia Hoagland, 1920 [28].
- All biramous parapodia have two postchaetal lobes. Proboscideal papillae have long, medium, or short stalks. 12– In the anterior parapodia, there is only one, medially inserted, slender triangular postchaetal lobe. The proboscideal papillae have short stalksGlycera macrobranchia Moore, 1911 [29].
- Proboscideal papillae have long stalks 13– Proboscideal papillae have medium-length or short stalks 14
- There are stalk without ridges and ailerons with pointed triangular basesGlycera alba Müller, 1776 [30].– There are stalks with numerous ridges and ailerons with triangular basesGlycera natalensis Day, 1967 [31].
- The proboscideal papillae have short stalks, the prostomium consists of about 11–15 rings, and ailerons have triangular basesGlycera tridactyla Schmarda, 1861 [12].– The proboscideal papillae have medium-length stalks, the prostomium consists of about 19–28 rings, and ailerons have pointed triangular basesGlycera africana Arwidsson, 1899 [32].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzo, A.E.; Steiner, T.M.; Amaral, A.C.Z. Glyceridae Grube 1850 (Annelida: Polychaeta) from southern and southeastern brazil, including a new species of Glycera. Biota Neotrop. 2007, 7, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Böggemann, M. Revision of the Glyceridae Grube 1850 (Annelida: Polychaeta). Abh. Senckenb. Naturforsch Ges. 2002, 555, 1–249. [Google Scholar]
- Böggemann, M.; Bienhold, C.; Gaudron, S.M. A new species of Glyceridae (Annelida: “Polychaeta”) recovered from organic substrate experiments at cold seeps in the eastern Mediterranean Sea. Mar. Biodivers. 2012, 42, 47–54. [Google Scholar] [CrossRef]
- Böggemann, M. Glyceriformia (Annelida) of the abyssal SW Atlantic and additional material from the SE Atlantic. Mar. Biodivers. 2016, 46, 227–241. [Google Scholar] [CrossRef]
- Choi, H.K.; Jung, T.W.; Yoon, S.M. A New Record of Glycerid Polychaete, Glycera fallax (Polychaeta: Glyceridae) from Korea. Korean J. Environ. Biol. 2015, 33, 274–278. [Google Scholar] [CrossRef]
- Sotomayor-Garcia, A.; Rueda, J.L.; Sánchez-Guillamón, O.; Vázquez, J.T.; Palomino, D.; Fernández-Salas, L.M.; López-González, N.; González-Porto, M.; Urra, J.; Santana-Casiano, J.M.; et al. Geomorphic features, main habitats and associated biota on and around the newly formed Tagoro submarine volcano, Canary Islands. In Seafloor Geomorphology as Benthic Habitat; Harris, P.T., Baker, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 51, pp. 835–846. [Google Scholar]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Ocean. Mar. Biol. Ann. Rev. 1979, 17, 193–284. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S. Macrobenthos of Intertidal zone of Halishahar Coast, Chittagong. Ph.D. Thesis, Department of Zoology, University of Chittagong, Chittagong, Bangladesh, 1990. [Google Scholar]
- Belaluzzaman, A.M. Ecology of the intertidal macrobenthic fauna in Cox’s Bazar coastal area, Bangladesh. Master’s Thesis, Institute of Marine Sciences and Fisheries, University of Chittagong, Chittagong, Bangladesh, 1995. [Google Scholar]
- Hossain, M.J.; Sarker, M.J.; Uddin, M.N.; Islam, A.; Tumpa, I.J.; Hossain, Z. Macrobenthos presence in the estuarine waters of the Meghna River, Ramghati, Laksmipur, Bangladesh. World Appl. Sci. J. 2018, 36, 598–604. [Google Scholar]
- Pramanik, M.N.; Chowdhury, S.H.; Kabir, S.M.H. Annelida. In Encyclopedia of Flora and Fauna of Bangladesh (Annelida, Echinodermata, Acanthocephala and Minor Phyla); Ahmad, M., Ahmed, A.T.A., Rahman, A.K.A., Ahmed, Z.U., Begum, Z.N.T., Hassan, M.A., Khondker, M., Eds.; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 2009; Volume 16, pp. 1–91. [Google Scholar]
- Schmarda, L.K. Neue wirbellose Thiere beobachtet und gesammelt auf einer Reise un die Erdr 1853 bis 1857: Erster Band (zweite halfte) Turbellarian, Rotatorien un Anneliden; Wilhelm Engelmann: Leipzig, Germany, 1861; pp. 1–164. [Google Scholar]
- Grube, A.E. Bemerkungen uber die familie der Glycereen. Schlesisch. Gesellsch. fur vaterlandisch. cultur Breslau Jahresber 1870, 47, 56–68. [Google Scholar]
- Lamarck, J.B. Histoire naturelle des Animaux sans Vertèbres, préséntant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent; precedes d’une Introduction offrant la determination des caracteres essentiels de l‘Animal, sa distinction du vegetal et desautres corps naturels, enfin; l’Exposition des Principes fondamentaux de la Zoologie: Paris, France, 1818; Volume 5, p. 612. [Google Scholar]
- Muir, A.I.; Hossain, M.M.M. The intertidal polychaete (Annelida) fauna of the Sitakunda coast (Chittagong, Bangladesh), with notes on the Capitellidae, Glyceridae, Lumbrineridae, Nephtyidae, Nereididae and Phyllodocidae of the “Northern Bay of Bengal Ecoregion”. ZooKeys 2014, 419, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.B.; Hutchings, P. Nephtys bangladeshi n. sp., a new species of Nephtyidae (Annelida: Phyllodocida) from Bangladesh coastal waters. Zootaxa 2016, 4079, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Flura, M.A.; Akhery, N.; Mohosena, B.T.; Masud, H.K. Physico-chemical and biological properties of water from the river Meghna, Bangladesh. Int. J. Fish. Aquac. Stud. 2016, 4, 161–165. [Google Scholar]
- Sharif, A.S.M.; Bakar, M.A.; Bhuyan, M.S. Assessment of water quality of the lower Meghna river estuary using multivariate analyses and RPI. Intl. J. Chem. Pharm. Technol. 2017, 2, 57–73. [Google Scholar]
- Syed, Z.H.; Choi, G.; Byeon, S. A numerical approach to predict water levels in ungauged regions—Case study of the meghna river estuary, Bangladesh. Water 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, M.M.H.; Hasan, M.M.; Bisshas, S.; Hossain, A.A.; Biswas, T.K. Fish biodiversity and their present conservation status in the Meghna River of Bangladesh. Int. J. Fish. Aquac. Stud. 2017, 5, 446–455. [Google Scholar]
- Grube, A.E. Die Familien der Anneliden. Archiv für Naturgeschichte Berlin 1850, 16, 249–364. [Google Scholar]
- Fiege, D.; Boggemann, M. Scanning electron microscopy of the proboscidial papillae of some European Glyceridae. Bull. Mar. Sci. 1997, 60, 559–563. [Google Scholar]
- World Polychaeta Database. Accessed through: World Register of Marine Species. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=129296 (accessed on 30 April 2020).
- Grube, A.E. Descriptiones Annulatorum novorum mare Ceylonicum habitantium ab honoratissimo Holdsworth collectorum. Proceed. Zool. Soci. London 1874, 325–329. [Google Scholar]
- Krishnamoorthi, B. Salinity tolerance and volume regulation in four species of polychaetes. Proceed. Indian Acad. Sci. 1962, 56, 363–371. [Google Scholar]
- Grube, A.E. Über eine Sammlung von wirbellosen Seethieren, welche Herr Dr. Eugen Reimann dem hiesigen zoologischen Museum zum Geschenk gemacht. Jahres-Bericht der Schlesiche Gesellschaft fuer vaterlandische Cultur. Breslau 1877, 54, 48–51. [Google Scholar]
- Hoagland, R.A. Polychaetous annelids collected by the United States fisheries steamer Albatross during the Philippine expedition of 1907–1909. Bull. U.S. Nat. Mus. 1920, 100, 603–635. [Google Scholar]
- McIntosh, W.C. Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Reports on the Scientific Results of the Voyage of HMS “Challenger”. Zoology 1885, 12, 1–554. [Google Scholar]
- Grube, A.E. Annulata Semperiana. Beiträge zur Kenntniss der Annelidenfauna der Philippinen nach den von Herrn Prof. Semper mitgebrachten Sammlungen. Mémoires l’Académie Impériale des Sciences de St. Pétersbourg 1878, 25, 1–300. [Google Scholar]
- Grube, A.E. Beschreibung neuer oder wenig bekannter Anneliden. Sechster Beitrag; Archiv für Naturgeschichte: Berlin, Germany, 1863; Volume 29, pp. 37–69. [Google Scholar]
- Grube, A.E. Reise der Österreichischen Frégatte Novara um die Erde inden Jahren 1857, 1858, 1859. Unter den Befehlen des Commodore B. von Wüllerstorf-Urbair. Novara-Expedition. Ser. Zoologischer Theil. 1867, 2, 1–46. [Google Scholar]
- Monatsberichte der Könglich Preussischen Akademie der Wissenschaften zu. Available online: https://www.biodiversitylibrary.org/page/35723826#page/581/mode/1up (accessed on 26 May 2020).





| Species | Type Locality | Data Source | Body Length, Width (Max) in mm, No. of Chaetigers (Holotype/Neotype) | Body Pigmentation | Prostomium Shape, No. of Rings, and No. of Prostomium Antenna | Proboscis Arrangement, and Type and Shape of Papillae |
|---|---|---|---|---|---|---|
| Glycera brevicirris Grube, 1870 [14] | Indonesia, Philippines, Indian Ocean | Based on Rizzo et al. [1] | 13 mm, 2 mm for 44 chaetigers (syntype) | Yellowish | Conical with 10 rings | Two types of papillae; type 1—numerous, digitiform, anteriorly smooth; and type 2—isolated, broader with longitudinal ridge |
| G. cinnamomea Grube, 1874 [25] | Sri Lanka, India | Based on original description | 94 mm, 3 mm at 75 chaetiger, 195 chaetigers (holotype) | White | Conical with 11 rings | Papillae short and digitiform |
| G. embranchiata Krishnamoorthi, 1962 [26] | India | Nomen dubium | - | - | ||
| G. lancadivae Schmarda, 1861 [13] | Sri Lanka | Based on original description | 50 mm, 3mm, 158 (holotype) | Yellowish-brown | Reversed cone, below the jaws. 16 larger | Hair-like papillae |
| G. manorae Fauvel, 1932 [27] | Manora Shoal, Karachi, India | Based on original description | 70 mm, 5 mm, n/a (holotype) | n/a | Acutely conical, with 10–12 rings and four tentacles | Small, cylindrical, unguiculate papillae |
| G. posterobranchia Hoagland, 1920 [28] | Marinduque Island, Philippines | Original | 75 mm, 3 mm, 90 (holotype) | Light brown | n/a, equal in length to first eight chaetigers | Two kinds of papillae: sucker-like and large blunt conical |
| G. rutilans Grube in McIntosh, 1885 [29] | Sri Lanka | Nomen nudum | ||||
| G. sagittariae McIntosh, 1885 [29] | Off Arrou Island, Madras coast, India | Based on original description | 110, 5, n/a | Not availavle (n/a) | n/a | Two types of papillae: short, globular or ovate; and long and slender without terminal nail- like appendage. |
| G. subaenea Grube, 1878 [30] | Philippines | Based on original description | 52 mm, 2.2 mm for 166 (holotype) segments | n/a | n/a | n/a |
| G. tesselata Grube, 1863 [31] | India | Based on original description | 48 mm, 6 mm, incomplete | n/a | Prostomium with 16–17 rings | n/a |
| Glycera sheikhmujibi n. sp. | Noakhali, Bangladesh | Based on present study | 42 mm and 2.2 mm for 158 segments | Whitish with black numerous black spot | Conical prostomium with 10–11 rings | Three types of papillae: type 1—numerous, digitiform with 2–3 ridges; type 2—isolated with one median straight ridge; and type 3—broader with a longitudinal ridge |
| Jaws and Aileron | Noto- & neuropodia; anteriror and posterior chaetigers | Branchiae | Chaetae | Remarks | ||
| Glycera brevicirris Grube, 1870 [14] | Aileron is triangular, with an elongated process on one side | Anterior parapodia uniramous with a pre- and postchaetal lobe where dorsal cirrus absent; posterior parapodia biramous with two triangular to digitiform prechaetal lobes | Absent | Parapodia with 2–4 simple capillary notochaetae and 5–13 spinigerous neurochaetae | ||
| G. cinnamomea Grube, 1874 [25] | n/a | n/a | ||||
| G. embranchiata Krishnamoorthi, 1962 [26] | Nomen dubium | Böggemann [2] reported as a nomen nudum | ||||
| G. lancadivae Schmarda, 1861 [13] | Jaws with large tooth and have a bent process | Parapodia biramous and tongue-like, but in the anterior segments cylindrical; dull, cone-shaped ventral cirrus in the first segments, pointed cone in the posterior segments | Branchiae absent in posterior segments | Few chaetae—curved and capillary, and partly spinigers | ||
| G. manorae Fauvel, 1932 [27] | Jaws obliquely truncated, aileron is triangular with an elongated process on one side | Parapodia with two sharp, triangular, equal anterior lobes and two similar but shorter blunt posterior lobes; dorsal cirrus elongated, knob near base of parapodia and ventral cirrus triangular | Retractile branchiae beginning at about the 17th foot | Dorsal capillary chaetae with narrow wings; ventral chaetae compound homogomph with finely serrated terminal piece. | ||
| G. posterobranchia Hoagland, 1920 [28] | Jaws with lateral appendages | Anterior parapodia with long, conical dorsal lobes; posterior parapodia elongated, divided into one dorsal and two ventral rami; dorsal cirrus a rounded tubercle and ventral cirrus similar shape to dorsal lobe | Branchiae begins at 25th chaetiger as small knobs at the dorsal base of parapodia | Two kinds: simple dorsal capillaries with finely serrated edges and ventral compounds with finely serrated blade | ||
| G. rutilans Grube in McIntosh, 1885 [29] | Nomen nudum | Böggemann [2] reported as a nomen nudum | ||||
| G. sagittariae Fauvel, 1932 [27] | Aileron with two long dagger-like processes | Parapodia with two equal elongated, tapering anterior lobes, and two equal, blunt, triangular posterior lobes; dorsal cirrus more or less remote | Present, simple and short beginning at 40th segment | n/a | ||
| G. subaenea Grube, 1878 [30] | n/a | Posterior parapodial lobes longer than the anterior ones; lower lobes triangular and wider than the upper ones, anterior lobes equally long, rounded | Branchiae present and positioned at the anterior wall of parapodium, separated into 2–3 fingerlike filaments, longer than ventral cirrus | n/a | ||
| G. tesselata Grube, 1863 [31] | n/a | n/a | n/a | n/a | ||
| Glycera sheikhmujibi n.sp. | Dark, hook-shaped jaws and ailerons with gently incised bases | Parapodia with digitiform prechaetal and postchaetal lobes; knob-like dorsal cirrus along the body and long ventral cirrus present posteriorly | Branchiae present, retractile, commencing from the 27th to 31st segments | 5–6 slender capillary notochaetae |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.B.; Hutchings, P. Glycera sheikhmujibi n. sp. (Annelida: Polychaeta: Glyceridae): A New Species of Glyceridae from the Saltmarsh of Bangladesh. Diversity 2020, 12, 213. https://doi.org/10.3390/d12060213
Hossain MB, Hutchings P. Glycera sheikhmujibi n. sp. (Annelida: Polychaeta: Glyceridae): A New Species of Glyceridae from the Saltmarsh of Bangladesh. Diversity. 2020; 12(6):213. https://doi.org/10.3390/d12060213
Chicago/Turabian StyleHossain, M. Belal, and Pat Hutchings. 2020. "Glycera sheikhmujibi n. sp. (Annelida: Polychaeta: Glyceridae): A New Species of Glyceridae from the Saltmarsh of Bangladesh" Diversity 12, no. 6: 213. https://doi.org/10.3390/d12060213