Abstract
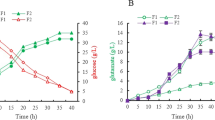
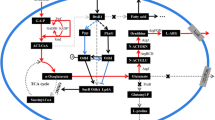
Lysine is widely used in food, medical and feed industries. The biosynthesis of l-lysine is closely related to NADPH level, but the regulation mechanism between the biosynthesis of l-lysine in C. glutamicum and the cofactor NADPH is still not clear. Here, a high intracellular NADPH level strain C. glutamicum XQ-5Δpgi::(zwf-gnd) was constructed by blocking the glycolytic pathway and overexpressing the pentose phosphate pathway in the lysine-producing strain C. glutamicum XQ-5, and the intracellular NADPH level in strain XQ-5Δpgi::(zwf-gnd) was increased from 3.57 × 10–5 nmol/(104 cells) to 1.8 × 10–4 nmol/(104 cell). Transcriptome analyses pointed to Cgl2680 as an important regulator of NADPH levels and l-lysine biosynthesis in C. glutamicum. By knocking out the gene Cgl2680, the intracellular NADPH level of the recombinant C. glutamicum lysCfbr ΔCgl2680 was raised from 7.95 × 10–5 nmol/(104 cells) to 2.04 × 10–4 nmol/(104 cells), consequently leading to a 2.3-fold increase in the NADPH/NADP+ ratio. These results indicated that the regulator Cgl2680 showed the negative regulation for NADPH regeneration. In addition, Cgl2680-deficient strain C. glutamicum lysCfbr ΔCgl2680 showed the increase of yield of both l-lysine and l-leucine as well as the increase of H2O2 tolerance. Collectively, our data demonstrated that Cgl2680 plays an important role in negatively regulating NADPH regeneration, and these results provides new insights for breeding l-lysine or l-leucine high-yielding strain.





Similar content being viewed by others
References
Belenky P, Bogan KL, Brenner C (2007) NAD metabolism in health and disease. Trends Biochem Sci 32:12–19
Bera AK, Ho NWY, Khan A et al (2011) A genetic overhaul of Saccharomyces cerevisiae 424A(LNH-ST) to improve xylose fermentation. J Ind Microbiol Biotechnol 38(5):617–626
Berger F, Ramirez-Hernandez MH, Ziegler M (2004) The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci 29:111–118
Brune I, Brinkrolf K, Kalinowski J, Pühler X, Tauch A (2005) The individual and common repertoire of DNA-binding transcriptional regulators of Corynebacterium glutamicum, Corynebacterium efficiens, Corynebacterium diphtheriae and Corynebacterium jeikeium deduced from the complete genome sequences. BMC Genomics 6:86
Cai DB et al (2017) A novel approach to improve poly-gamma-glutamic acid production by NADPH Regeneration in Bacillus licheniformis WX-02. Sci Rep 7:43404. https://doi.org/10.1038/srep43404
Celton M, Sanchez I, Goelzer A et al (2012a) A comparative transcriptomic, fluxomic and metabolomic analysis of the response of Saccharomyces cerevisiae to increases in NADPH oxidation. Bmc Genomics 13(1):317
Celton M, Goelzer A, Camarasa C, Fromion V, Dequin S (2012b) A constraint-based model analysis of the metabolic consequences of increased NADPH oxidation in Saccharomyces cerevisiae. Metab Eng 14:366–379
Chen XL, Liu J, Luo QL et al (2017) Manipulation of cofactor balance in microorganisms. Chin J Biotech 33(1):16–26
Chen C, Zhang Y, Lei Xu et al (2018) Transcriptional control of the phenol hydroxylase gene phe of Corynebacterium glutamicum by the AraC-type regulator PheR. Microbiol Res 209:14–20
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31(1):20–28
de Graef MR, Alexeeva S, Snoep JL et al (1999) The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol 181(8):2351–2357
Ehsani M, Fernandez MR, Biosca JA, Dequin S (2009) Reversal of coenzyme specificity of 2,3-butanediol dehydrogenase from Saccharomyces cerevisiae and in vivo functional analysis. Biotechnol Bioeng 104:381–389
Fu X, Li P, Zhang L, Li S (2019) Understanding the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation based on integration of RNA-Seq and metabolite data. Appl Microbiol Biotechnol 103(6):2715–2729
Heux S, Cachon R, Dequin S (2006) Cofactor engineering in Saccharomyces cerevisiae: expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab Eng 8(4):303–314
Hou J, Lages NF, Oldiges M et al (2009) Metabolic impact of redox cofactor perturbations in Saccharomyces cerevisiae. Metab Eng 11(4/5):253–261
Jan J, Martinez I, Wang YP et al (2013) Metabolic engineering and transhydrogenase effects on NADPH availability in Escherichia coli. Biotechnol Prog 29(5):1124–1130
Juhnke H, Krems B, Entian KD et al (1996) Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Mol Genet Genomics 252:456–464
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium. glutamicum molecular analysis of the ilvb-ilvc operon. J Bacteriol 175:5595–5603
Khosla C, Keasling JD (2003) Metabolic engineering for drug discovery and development. Nat Rev Drug Discov 2(12):1019–1025
Kiefer P, Heinzle E, Zelder O, Wittmann C (2004) Comparative metabolic flux analysis of lysine-producing Corynebacterium glutamicum cultured on glucose or fructose. Appl Env Microbiol 70:229–239
Kletzien RF, Harris PK, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J 8:174–181
Kuge T, Teramoto H, Inui M (2015) AraR, an L-arabinose-responsive transcriptional regulator in Corynebacterium glutamicum ATCC 31831. Exerts different degrees of repression depending on the location of its binding sites within the three target promoter regions. J Bacteriol 197(24):3788–3796
Laganowsky A, Reading E, Allison TM et al (2014) Membrane proteins bind lipids selectively to modulate their structure and function. Nature 510:172–175
Lee SH, Jo SH, Lee SM, Koh HJ, Song H, Park JW, Lee WH, Huh TL (2004) Role of NADP+-dependent isocitrate dehydrogenase (NADP+-ICDH) on cellular defence against oxidative injury by gamma-rays. Int J Radiat Biol 80:635–642
Lee WH, Kim JW, Park EH et al (2013) Effects of NADH kinase on NADPH-dependent biotransformation processes in Escherichia coli. Appl Microbiol Biotechnol 97(4):1561–1569
Leßmeier L, Pfeifenschneider J, Carnicer M et al (2015) Production of carbon-13-labeled cadaverine by engineered Corynebacterium glutamicum using carbon-13-labeled methanol as co-substrate. Appl Microbiol Biotechnol 99(23):10163–10176
Maeng O, Kim YC, Shin HJ, Lee JO, Huh TL, Kang KI, Kim YS, Paik SG, Lee H (2004) Cytosolic NADP+-dependent isocitrate dehydrogenase protects macrophages from LPS-induced nitric oxide and reactive oxygen species. Biochem Biophys Res Commun 317:558–564
Mols M, van Kranenburg R, van Melis CC, Moezelaar R, Abee T (2010) Analysis of acid-stressed Bacillus cereus reveals a major oxidative response and inactivation-associated radical formation. Environ Microbiol 12(4):873–885
Moritz B, Striegel K, de Graaf AA, Sahm H (2000) Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose phosphate pathway flux in vivo. FEBS J 267:3442–3452
Nagai Y, Ito H, Yasueda H (2010) Amino acid production: l-lysine. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology, vol 7. Wiley, Hoboken, pp 1–10
Ohnishi J, Mitsuhashi S, Hayashi M, Ando S, Yokoi H, Ochiai K, Ikeda M (2002) A novel methodology employing Corynebacterium glutamicum genome information to generate a new l-lysine-producing mutant. Appl Microbiol Biotechnol 58:217–223
Ouyang L, Holland P, Lu H, Bergenholm D, Nielsen J (2018) Integrated analysis of the yeast NADPH-regulator Stb5 reveals distinct differences in NADPH requirements and regulation in different states of yeast metabolism. FEMS Yeast Res 18:091. https://doi.org/10.1093/femsyr/foy091
Pollak N, Dolle C, Ziegler M (2007) The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem J 402:205–218
Qin Y, Dong ZY, Liu LM et al (2009) Manipulation of NADH metabolism in industrial strains. Chin J Biotech 25(2):161–169
Shi F, Kawai S, Mori S et al (2005) Identification of ATP-NADH kinase isozymes and their contribution to supply of NADP(H) in Saccharomyces cerevisiae. FEBS J 272:3337–3349
Singh R, Lemire J, Mailloux RJ, Appanna VD (2008) A novel strategy involved anti-oxidative defense: the conversion of NADH into NADPH by a metabolic network. PLoS ONE 3:e2682. https://doi.org/10.1371/journal.pone.0002682
Spaans SK, Weusthuis RA, John VDO et al (2015) NADPH-generating systems in bacteria and archaea. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00742
Sánchez AM, Andrews J, Hussein I et al (2006) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog 22(2):420–425
Takeno S, Hori K, Ohtani S, Mimura A, Mitsuhashi S, Ikeda M (2016) L-Lysine production independent of the oxidative pentose phosphate pathway by Corynebacterium glutamicum with the Streptococcus mutans gapN gene. Metab Eng 37:1–10
Vadali RV, Bennett GN, San KY (2004) Cofactor engineering of intracellular CoA/acetyl-CoA and its effect on metabolic flux redistribution in Escherichia coli. Metab Eng 6(2):133–139
Wan F, Yin J, Sun W, Gao H (2019) Oxidized OxyR up-regulates ahpCF expression to suppress plating defects of oxyR- and catalase-deficient strains. Front Microbiol 10:439
Wang T, Gao F, Kang Y, Zhao C, Su T, Li M, Si M, Shen X (2016) Mycothiol peroxidase MPx protects Corynebacterium glutamicum against acid stress by scavenging ROS. Biotechnol Lett 38(7):1221–1228
Witthoff S, Muhlroth A, Marienhagen J et al (2013) C1 Metabolism in Corynebacterium glutamicum: an endogenous pathway for oxidation of methanol to carbon dioxide. Appl Environ Microbiol 79(22):6974–6983
Wittmann C, de Graaf A (2005) Metabolic flux analysis in Corynebacterium glutamicum. CRC Press, Boca Raton (FL)
Wittmann C, Heinzle E (2002) Genealogy profiling through strain improvement by using metabolic network analysis: metabolic flux genealogy of several generations of lysine-producing corynebacteria. Appl Env Microbiol 68:5843–5859
Xu J (2014) Breeding of Corynebacterium glutamicum L-lysine-producing bacteria based on metabolic engineering [D]: [PhD thesis]. College of Biotechnology, Jiangnan University, Wuxi
Xu J-Z, Yang H-K, Zhang W-G (2018) NADPH metabolism: a survey of its theoretical characteristics and manipulation strategies in amino acid biosynthesis. Crit Rev Biotechnol 38(7):1061–1071
Xu W, Yao J, Liu L, Ma X, Li W, Sun X, Wang Y (2019) Improving squalene production by enhancing the NADPH/NADP(+) ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol Biofuels. https://doi.org/10.1186/s13068-019-1415-x
Yang Z, Zhang H, Hung HC, Kuo CC, Tsai LC, Yuan HS, Chou WY, Chang GG, Tong L (2002) Structural studies of the pigeon cytosolic NADP(+)-dependent malic enzyme. Protein Sci 11:332–341
Yeom S, Yeom J, Park W (2010) NtrC-sensed nitrogen availability is important for oxidative stress defense in Pseudomonas putida KT2440. J Microbiol 48:153–159. https://doi.org/10.1007/s12275-010-0075-0
Ying WH (2008) NAD(+)/NADH and NADP(+)/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Sign 10:179–206
Zelder O, Pompejus M, Schroder H, Kroger B, Klopprogge C, Haberhauer G (2005) Genes coding for G6P dehydrogenase proteins. US Patent No. WO03042389
Zhan M, Kan B, Dong J, Guochao Xu, Han R, Ni Ye (2019) Metabolic engineering of Corynebacterium glutamicum for improved L-arginine synthesis by enhancing NADPH supply. J Ind Microbiol Biotechnol 46:45–54
Zhang J, Fu RY, Hugenholtz J, Li Y, Chen J (2007) Glutathione protects Lactococcus lactis against acid stress. Appl Environ Microbiol 73(16):5268–5275. https://doi.org/10.1128/AEM.02787-06
Zhang Y, Smallbone LA, diCenzo GC, Morton R, Finan TM (2016) Loss of malic enzymes leads to metabolic imbalance and altered levels of trehalose and putrescine in the bacterium Sinorhizobium meliloti. BMC Microbiol 16:163. https://doi.org/10.1186/s12866-016-0780-x
Zhou X (2012) Research on L-valine metabolic engineering breeding [D]: [Master Degree Thesis]. College of Biological Engineering, Jiangnan University, Wuxi
Acknowledgements
This work was supported by the National Natural Science Foundation of China [No. 31601459], the China Postdoctoral Science Foundation [No. 2016M590410], the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions, the 111 Project [No. 111–2-06].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Yu, H., Xu, J. et al. Deciphering the crucial roles of AraC-type transcriptional regulator Cgl2680 on NADPH metabolism and l-lysine production in Corynebacterium glutamicum. World J Microbiol Biotechnol 36, 82 (2020). https://doi.org/10.1007/s11274-020-02861-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02861-y