Abstract
Purpose:
Augmented reality can improve the outcome of hepatic surgeries, assuming an accurate liver model is available to estimate the position of internal structures. While researchers have proposed patient-specific liver simulations, very few have addressed the question of boundary conditions. Resulting mainly from ligaments attached to the liver, they are not visible in preoperative images, yet play a key role in the computation of the deformation.
Method:
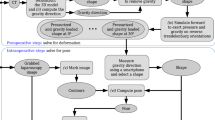
We propose to estimate both the location and stiffness of ligaments by using a combination of a statistical atlas, numerical simulation, and Bayesian inference. Ligaments are modeled as polynomial springs connected to a liver finite element model. They are initialized using an anatomical atlas and stiffness properties taken from the literature. These characteristics are then corrected using a reduced-order unscented Kalman filter based on observations taken from the laparoscopic image stream.
Results:
Our approach is evaluated using synthetic data and phantom data. By relying on a simplified representation of the ligaments to speed up computation times, it is not estimating the true characteristics of ligaments. However, results show that our estimation of the boundary conditions still improves the accuracy of the simulation by 75% when compared to typical methods involving Dirichlet boundary conditions.
Conclusion:
By estimating patient-specific boundary conditions, using tracked liver motion from RGB-D data, our approach significantly improves the accuracy of the liver model. The method inherently handles noisy observations, a substantial feature in the context of augmented reality.








Similar content being viewed by others
References
Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in europe in 2008. Eur J Cancer 46(4):765–781
Orcutt ST, Anaya DA (2018) Liver resection and surgical strategies for management of primary liver cancer. J Moffitt Cancer Center 25(1):1073274817744621
Puerto-Souza GA, Mariottini GL (2013) Toward long-term and accurate augmented-reality display for minimally-invasive surgery. In: IEEE ICRA, pp 5384–5389
Chen L, Tang W, John NW (2017) Real-time geometry-aware augmented reality in minimally invasive surgery. Healthc Technol Lett 4(5):163–167
Haouchine N, Dequidt J, Peterlik I, Kerrien E, Berger M-O, Cotin S (2013) Image-guided simulation of heterogeneous tissue deformation for augmented reality during hepatic surgery. In: 2013 IEEE ISMAR, pp 199–208
Suwelack S (2015) Real-time Biomechanical Modeling for Intraoperative Soft Tissue Registration. Ph.D. thesis, Karlsruhe Institute of Technology
Peterlík I, Courtecuisse H, Rohling R, Abolmaesumi P, Nguan C, Cotin S, Salcudean S (2018) Fast elastic registration of soft tissues under large deformations. Med Image Anal 45:24–40
Clements LW, Collins JA, Weis JA, Simpson AL, Kingham TP, Jarnagin WR, Miga MI (2017) Deformation correction for image guided liver surgery: an intraoperative fidelity assessment. Surgery 162(3):537–547
Chui C-K, Kobayashi E, Chen X, Hisada T, Sakuma I (2007) Transversely isotropic properties of porcine liver tissue: experiments and constitutive modelling. Med Biol Eng Comput 45(1):99–106
Marchesseau S, Chatelin S, Delingette H (2017) Nonlinear biomechanical model of the liver. In: Biomechanics of living organs, vol 1, Elsevier, Amsterdam, pp 243–265
Peterlík I, Duriez C, Cotin S (2012) Modeling and real-time simulation of a vascularized liver tissue. In: MICCAI, pp. 50–57
Plantefève R, Peterlik I, Courtecuisse H, Trivisonne R, Radoux J-P, Cotin S (2014) Atlas-based transfer of boundary conditions for biomechanical simulation. In: MICCAI, pp 33–40
Ozkan E, Goksel O (2018) Compliance boundary conditions for patient-specific deformation simulation using the finite element method. Biomed Phys Eng 4(2):025003
Peterlik I, Courtecuisse H, Duriez C, Cotin S (2014) Model-based identification of anatomical boundary conditions in living tissues. In: IPCAI, pp 196–205
Largillière F, Coevoet E, Sanz-Lopez M, Grisoni L, Duriez C (2016) Stiffness rendering on soft tangible devices controlled through inverse fem simulation. In: Proceedings of IROS, pp 5224–5229
Gray H (1918) Anatomy of the human body. Lea & Febiger, Philadelphia
Abdel-Misih SRZ, Bloomston M (2010) Liver anatomy. Surg Clin N Am 90(4):643–653
Delingette H, Ayache N (2004) Soft tissue modeling for surgery simulation. In: Computational models for the human body, vol 12, Elsevier, Amsterdam, pp 453–550
Nava A, Mazza E, Kleinermann F, Avis NJ, McClure J, Bajka M (2004) Evaluation of the mechanical properties of human liver and kidney through aspiration experiments. Technol Health Care 12(3):269–280
Rodriguez Lelis J, Médez Aguirre J, Arellano Cabrera J, Navarro Torres J, Pliego A (2011) On the mechanical design of cruciate ligaments based on polymeric ropes for knee prosthesis. Revista Mexicana de Ingenieria biomédica 32(1):25–31
White EJ, Cunnane EM, McMahon M, Walsh MT, Coffey JC, O’Sullivan L (2018) Mechanical characterisation of porcine non-intestinal colorectal tissues for innovation in surgical instrument design. J Eng Med 232(8):796–806
Sarac TP, Carnevale K, Smedira N, Tanquilut E, Augustinos P, Patel A, Naska T, Clair D, Ouriel K (2005) In vivo and mechanical properties of peritoneum/fascia as a novel arterial substitute. J Vasc Surg 41(3):490–497
Reese SP, Ellis BJ, Weiss JA (2013) Multiscale modeling of ligaments and tendons. Springer, New York, pp 103–147
Witz CA, Montoya-Rodriguez IA, Cho S, Centonze VE, Bonewald LF, Schenken RS (2001) Composition of the extracellular matrix of the peritoneum. J Soc Gynecol Invest 8(5):299–304
Julier SJ, Uhlmann JK, Durrant-Whyte HF (1995) A new approach for filtering nonlinear systems. In: Proceedings of ACC’95, vol 3, pp 1628–1632
Julier SJ, Uhlmann JK (2002) Reduced sigma point filters for the propagation of means and covariances through nonlinear transformations. In: Proceedings of the American Control Conference, vol 2, pp 887–892. https://doi.org/10.1109/ACC.2002.1023128
Peterlik I, Haouchine N, Ručka L, Cotin S (2017) Image-driven stochastic identification of boundary conditions for predictive simulation. In: MICCAI, pp 548–556
Barbič J, James D (2005) Real-time subspace integration for st. venant-kirchhoff defromable models. ACM Trans Graph 24(3):982–990
Forestiero A, Carniel EL, Natali AN (2014) Biomechanical behaviour of ankle ligaments: constitutive formulation and numerical modelling. Comput Methods Biomech Biomed Eng 17(4):395–404
Maas SA, Ellis BJ, Ateshian GA, Weiss JA (2012) Febio: finite elements for biomechanics. J Biomech Eng 134(1)
Lu Y-C, Kemper A, Gayzik S, Untaroiu C, Beillas P (2013) Statistical modeling of human liver incorporating the variations in shape, size, and material properties. In: 57th Stapp car crash conference, vol 57, pp 285–311
Allard J, Cotin S, Faure F, Bensoussan P-J, Poyer F, Duriez C, Delingette H, Grisoni L (2007) SOFA: an open source framework for medical simulation, vol 125. IOS Press, New York, pp 13–18
Bouguet J-Y (1999) Pyramidal implementation of the lucas kanade feature tracker. Intel Corporation, Microprocessor Research Labs, pp 1–10
Funding
This study was funded from the EU H2020 Research and Innovation programme under Marie Sklodowska-Curie Grant Agreement No. 722068.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This articles does not contain patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikolaev, S., Cotin, S. Estimation of boundary conditions for patient-specific liver simulation during augmented surgery. Int J CARS 15, 1107–1115 (2020). https://doi.org/10.1007/s11548-020-02188-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-020-02188-x