Abstract
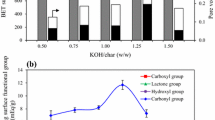
Activated charcoal was prepared from Acrocomia aculeata (macaúba) endocarp by ZnCl2 activation and then used for the adsorptive purification of pretreated crude glycerol (CG) containing pigments, such as β-carotene. The pretreatment of glycerol involved filtration of the K3PO4 formed by the addition of H3PO4 to the crude glycerol containing KOH. A mixture of 1.38:1 w/w of ZnCl2:Acrocomia aculeata pulp was heated at 120 °C with stirring for 24 h. The mixture was activated by heating at 600 °C for 3 h. The activated charcoal was cooled to 25 °C, washed with a 1:1 mixture of methanol and water (100 mL) and heated at 150 °C for 2 h. The surface properties of the activated charcoal (surface area 627 m2 g−1, pore volume 0.39 m3 g−1, Bronsted sites 118.23 μmol g−1, and Lewis sites 104.86 μmol g−1) and the adsorption capacity for impurities in H3PO4-pretreated crude glycerol were investigated. The activated charcoal exhibited the most suitable surface properties for the purification of pretreated crude glycerol, attaining a 95.99% glycerol concentration (by GC) using 10 g/L with gravity filtration through a column at room temperature over a 48-h period. The purified glycerol was characterized by GC/MS, 1H- and 13C-NMR, and DSC and TG analyses. The activated charcoal was regenerated by washing with methanol and hexane and heating to 150 °C for 5 h. The activated charcoal could be re-used three times to remove all of the pigments before it was necessary to re-activate the charcoal by heating with ZnCl2.





Similar content being viewed by others
References
Zahan KA, Kano M (2017) biodiesel production from palm oil, its by-products, and mill effluent: a review. Energies. https://www.mdpi.com/1996-1073/11/8/2132/pdf
Wang T (2019) Global biodiesel production by country. Statista. https://www.statista.com/statistics/274168/biofuel-production-in-leading-countries-in-oil-equivalent/
Knoema Enterprise Data Solutions (2017) Biodiesel production. https://knoema.com/atlas/topics/Energy/ Renewables/Biodiesel-production. Accessed 10 Apr 2020
United States Energy Information Administration (2020) Biodiesel Production by Country. https://www.indexmundi.com/energy/?product=biodiesel&graph=production&display=rank
Gupta M, Kumar N (2012) Scope and opportunities of using glycerol as an energy source. Renew Sust Energ Rev 16:4551–4556. https://doi.org/10.1016/j.rser.2012.04.001
Quispe CAG, Coronado CJR, Carvalho JA Jr (2013) Glycerol: production, consumption, prices, characterization and new trends in combustion. Renew Sust Energ Rev 27:475–493. https://doi.org/10.1016/j.rser.2013.06.017
Baba Y, Tada C, Watanabe R, Fukuda Y, Chida N, Nakai Y (2013) Anaerobic digestion of crude glycerol from biodiesel manufacturing using a large-scale pilot plant: methane production and application of digested sludge as fertilizer. Bioresour Technol 140:342–348. https://doi.org/10.1016/j.biortech.2013.04.020
Surendra KC, Sawatdeenarunat C, Shrestha S, Sung S, Khanal SK (2015) Anaerobic digestion-based biorefinery for bioenergy and biobased products. Ind Biotechnol 11:103–112. https://doi.org/10.1089/ind.2015.0001
Zijlstra RT, Beltranena E (2013) Swine convert co-products from food and biofuel industries into animal protein for food. Animal Frontiers 3:48–53. https://doi.org/10.2527/af.2013-0014
González R, Smith R, Blanco D, Fierro J, Gómez X (2019) Application of thermal analysis for evaluating the effect of glycerine addition on the digestion of swine manure. J Therm Anal Calorim 135:2277–2286. https://doi.org/10.1007/s10973-018-7464-8
Pott RWM, Howe CJ, Dennis JS (2014) The purification of crude glycerol derived from biodiesel manufacture and its use as a substrate by Rhodopseudomonas palustris to produce hydrogen. Bioresour Technol 152:464–470. https://doi.org/10.1016/j.biortech.2013.10.094
Mota CJA, Peres Pinto B, de Lima AL (2017) A versatile renewable feedstock for the chemical industry. Chapter 2. Glycerol utilization. https://www.springer.com/gp/book/9783319593746
Vivek N, Pandey A, Binod P (2015) Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.02.020
Isahak WNRW, Ismail M, Yarmo MA, Jahim JM, Salimon J (2010) Purification of crude glycerol from transesterification RBD palm oil over homogeneous and heterogeneous catalysts for the biolubricant preparation. J Appl Sci 10:2590–2595. https://doi.org/10.3923/jas.2010.2590.2595
Konstantinovic S, Danilovic B, Ciric J et al (2016) Valorization of crude glycerol from biodiesel production. Chem Ind Chem Eng Q 22:461–489. https://doi.org/10.2298/CICEQ160303019K
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol Biofuels 5:13. https://doi.org/10.1186/1754-6834-5-13
Chen J, Yan S, Zhang X, Tyagi RD, Surampalli RY, Valéro JR (2018) Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manag 71:164–175. https://doi.org/10.1016/j.wasman.2017.10.044
García-Martín JF, Alés-Álvarez FJ, Torres-García M, Feng C-H, Álvarez-Mateos P (2019) Production of oxygenated fuel additives from residual glycerine using biocatalysts obtained from heavy-metal-contaminated Jatropha curcas L. Roots. Energies. https://www.mdpi.com/1996-1073/12/4/740, Production of Oxygenated Fuel Additives from Residual Glycerine Using Biocatalysts Obtained from Heavy-Metal-Contaminated Jatropha curcas L. Roots
Manosak R, Limpattayanate S, Hunsom M (2011) Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process Technol 92:92–99. https://doi.org/10.1016/j.fuproc.2010.09.002
Delample M, Villandier N, Douliez J-P, Camy S, Condoret J-S, Pouilloux Y, Barrault J, Jerôme F (2010) Glycerol as a cheap, safe and sustainable solvent for the catalytic and regioselective β, β-diarylation of acrylates over palladium nanoparticles. Green Chemistry. https://pubs.rsc.org/en/Content/ArticleLanding/GC/2010/B925021B#!divAbstract
Hansen CF, Hernandez A, Mullan BP, Moore K, Trezona-Murray M, King RH, Pluske JR (2009) A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim Prod Sci 49:154. https://doi.org/10.1071/ea08210
Thompson JC, He BB (2006) Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl Eng Agric 22:261–265. https://doi.org/10.13031/2013.20272
Frimmel FH, Noble RD, Terry PA (2005) Principles of chemical separations with environmental applications. Angew Chem 117:187–188. https://doi.org/10.1002/ange.200485210
Van Gerpen J (2005) Biodiesel processing and production. Fuel Process Technol 86:1097–1107. https://doi.org/10.1016/j.fuproc.2004.11.005
Dzyazko YS, Rozhdestvenska LM, Vasilyuk SL, Kudelko KO, Belyakov VN (2017) Composite membranes containing nanoparticles of inorganic ion exchangers for electrodialytic desalination of glycerol. Nanoscale Res Lett 12:438. https://doi.org/10.1186/s11671-017-2208-4
Isahak WNRW, Ismail M, Yarmo MA, Jahim JM, Salimon J (2010) Purification of crude glycerol from transesterification RBD palm oil over homogeneous and heterogeneous catalysts for the biolubricant preparation. Journal of Applied Sciences. https://scialert.net/abstract/?doi=jas.2010.2590.2595, Purification of Crude Glycerol from Transesterification RBD Palm Oil over Homogeneous and Heterogeneous Catalysts for the Biolubricant Preparation
Carmona M, Valverde JL, Perez A, Warcholb J, Rodrigueza JF (2009) Purification of glycerol/water solutions from biodiesel synthesis by ion exchange: sodium removal Part I. J Chem Technol; Biotechnol. https://doi.org/10.1002/jctb.2106
Saleh J, Tremblay AY, Dubé MA (2010) Glycerol removal from biodiesel using membrane separation technology. Fuel. 89:2260–2266. https://doi.org/10.1016/j.fuel.2010.04.025
Kongjao S, Damronglerd S, Hunsom M (2010) Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J Chem Eng 27:944–949. https://doi.org/10.1007/s11814-010-0148-0
Isahak W, Che Ramli ZA, Ismail M, et al (2014) Recovery and purification of crude glycerol from vegetable oil transesterification: a review. Sep Purif Rev. https://doi.org/10.1080/15422119.2013.851696
Xiao Y, Xiao G, Varma A, Isahak WW (2013) A universal procedure for crude glycerol purification from different feedstocks in biodiesel production: experimental and simulation study. Ind Eng Chem Res 52:14291–14296. https://doi.org/10.1021/ie402003u
Ardi MS, Aroua MK, Hashim NA (2015) Progress, prospect and challenges in glycerol purification process: a review. Renew Sust Energ Rev 42:1164–1173. https://doi.org/10.1016/j.rser.2014.10.091
Turk Sekulić M, Pap S, Stojanović Z, Bošković N, Radonić J, Šolević Knudsen T (2018) Efficient removal of priority, hazardous priority and emerging pollutants with Prunus armeniaca functionalized biochar from aqueous wastes: experimental optimization and modeling. Sci Total Environ 613-614:736–750. https://doi.org/10.1016/j.scitotenv.2017.09.082
Alves CCO, Faustino MV, Franca AS, Oliveira LS (2014) Comparative evaluation of activated carbons prepared by thermo-chemical activation of lignocellulosic residues in fixed bed column studies. Int J Eng Technol 7:465–469. https://doi.org/10.7763/ijet.2015.v7.838
Yakout SM, Sharaf El-Deen G (2016) Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab J Chem 9:S1155–S1162. https://doi.org/10.1016/j.arabjc.2011.12.002
Zhu Z, Liu Z, Liu S, Niu H, Hu T, Liu T, Xie Y (2000) NO reduction with NH 3 over an activated carbon-supported copper oxide catalysts at low temperatures. Appl Catal B Environ 26:25–35. https://doi.org/10.1016/S0926-3373(99)00144-7
Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere. 149:168–176. https://doi.org/10.1016/j.chemosphere.2016.01.093
Elmouwahidi A, Zapata-Benabithe Z, Carrasco-Marín F, Moreno-Castilla C (2012) Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour Technol 111:185–190. https://doi.org/10.1016/j.biortech.2012.02.010
Ubago-Pérez R, Carrasco-Marín F, Fairén-Jiménez D, Moreno-Castilla C (2006) Granular and monolithic activated carbons from KOH-activation of olive stones. Microporous Mesoporous Mater 92:64–70. https://doi.org/10.1016/j.micromeso.2006.01.002
Byamba-Ochir N, Shim WG, Balathanigaimani MS, Moon H (2016) Highly porous activated carbons prepared from carbon rich Mongolian anthracite by direct NaOH activation. Appl Surf Sci 379:331–337. https://doi.org/10.1016/j.apsusc.2016.04.082
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285. https://doi.org/10.1016/j.ecoenv.2017.01.010
Ternero-Hidalgo JJ, Rosas JM, Palomo J, Valero-Romero MJ, Rodríguez-Mirasol J, Cordero T (2016) Functionalization of activated carbons by HNO3 treatment: influence of phosphorus surface groups. Carbon N Y 101:409–419. https://doi.org/10.1016/j.carbon.2016.02.015
Shamsuddin MS, Yusoff NRN, Sulaiman MA (2016) Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chem 19:558–565. https://doi.org/10.1016/j.proche.2016.03.053
Giraldo L, Ladino Y, Piraján JCM, Rodríguez MP (2007) Synthesis and characterization of activated carbon fibers from Kevlar. Ecletica Quim 32:55–62. https://doi.org/10.1590/S0100-46702007000400008
Yorgun S, Yildiz D (2015) Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J Taiwan Inst Chem Eng 53:122–131. https://doi.org/10.1016/j.jtice.2015.02.032
Kumar A, Jena HM (2017) Adsorption of Cr(VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Saf Environ Prot 109:63–71. https://doi.org/10.1016/j.psep.2017.03.032
Demiral İ, Aydın Şamdan C, Demiral H (2016) Production and characterization of activated carbons from pumpkin seed shell by chemical activation with ZnCl2. Desalin Water Treat 57:2446–2454. https://doi.org/10.1080/19443994.2015.1027276
Khenniche L, Benissad-Aissani F (2010) Adsorptive removal of phenol by coffee residue activated carbon and commercial activated carbon: equilibrium, kinetics, and thermodynamics. J Chem Eng Data 55:4677–4686. https://doi.org/10.1021/je100302e
Komintarachat C, Chuepeng S (2010) Methanol-based transesterification optimization of waste used cooking oil over potassium hydroxide catalyst. Am J Appl Sci https://pdfs.semanticscholar.org/407c/0888dd132d6b831086d581a6d1153d5d778c.pdf 7:1073–1078
Barbosa SL, Ottone M, Santos MC, Junior GC, Lima CD, Glososki GC, Lopes NP, Klein SI (2015) Benzyl benzoate and dibenzyl ether from benzoic acid and benzyl alcohol under microwave irradiation using a SiO2-SO3H catalyst. Catal Commun 68:97–100. https://doi.org/10.1016/j.catcom.2015.04.033
Moreno-Castilla C (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon. 42:83–94. https://doi.org/10.1016/j.carbon.2003.09.022
Marques MBF, Araujo BCR, Fernandes C, Yoshida MI, Mussel WN, Sebastião RCO (2020) Kinetics of lumefantrine thermal decomposition employing isoconversional models and artificial neural network. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20190211
Brito LG, Leite GQ, Duarte FÍC, Ostrosky EA, Ferrari M, de Lima AAN, Nogueira FHA, Aragão CFS, de Lelis Ferreira BD, de Freitas Marques MB, Yoshida MI, Mussel WN, Sebastiao RCO, Gomes APB (2019) Thermal behavior of ferulic acid employing isoconversional models and artificial neural network. J Therm Anal Calorim 138:3715–3726. https://doi.org/10.1007/s10973-019-08114-x
Ferreira BDL, Araújo NRS, Ligório RF, Pujatti FJP, Mussel WN, Yoshida MI, Sebastião RCO (2018) Kinetic thermal decomposition studies of thalidomide under non-isothermal and isothermal conditions. J Therm Anal Calorim 134:773–782. https://doi.org/10.1007/s10973-018-7568-1
Funding
The authors acknowledge the financial support and scholarships furnished by the CNPq, FAPEMIG and financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barbosa, S.L., de Freitas, M.S., dos Santos, W.T.P. et al. Preparation of activated charcoal from Acrocomia aculeata for purification of pretreated crude glycerol. Biomass Conv. Bioref. 12, 2441–2449 (2022). https://doi.org/10.1007/s13399-020-00745-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00745-7