Abstract
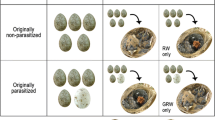
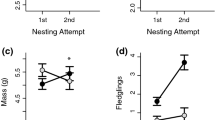
The co-evolutionary arms race between brood parasites and their hosts involves stepwise adaptive changes on the side of the parasites as well as hosts. In response to avian brood parasitism, host females may eject a parasitic egg, bury the parasitized clutch or desert it. After nest desertion, females commonly re-nest and may move further to avoid being parasitized again. Here we tested whether and under which conditions the within-season re-nesting prevents brood parasitism in the great reed warbler (Acrocephalus arundinaceus). We analysed 78 re-nesting events of 58 naturally parasitized host females that deserted their nests in response to the common cuckoo (Cuculus canorus) parasitism. The parasitism rate in the replacement nests of these females was 60%. Most of these females built their replacement nests less than 143 m from the previous nests. The probability for replacement nests to be parasitized increased with increasing instantaneous parasitism rate but not with the re-nesting distance or timing of the replacement clutch. We explain this by the high level of cuckoo parasitism across the whole study site during the major part of the breeding season. To better understand the patterns and consequences of host re-nesting behaviour, further studies in other host populations with different levels of cuckoo parasitism would be desirable.
Significance statement
Although various factors affecting avian breeding dispersal have been studied, little is known about the relationship between the within-season re-nesting distances and fate of replacement nests. Moreover, there is a lack of studies focusing on the consequences of re-nesting dispersal in response to brood parasitism and, to our best knowledge, this is the first study investigating this topic in a host of an evictor parasite.



Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. The custom R code generated during the current study is available from the corresponding author on reasonable request.
References
Abernanthy VE, Langmore NE (2017) The first stages of coevolution between a brood parasite and its new host: are naïve hosts defenceless? Emu 117:114–129
Adamík P, Hušek J, Cepák J (2009) Rapid decline of common cuckoo Cuculus canorus parasitism in red-backed shrikes Lanius collurio. Ardea 97:17–22
Alvarez F (2003) Parasitism rate by the common cuckoo Cuculus canorus increases with high density of host’s breeding pairs. Ornis Fenn 80:193–196
Bates D, Maechler M, Bolker B, Walker S (2016) Linear mixed-effects models using ‘Eigen’ and S4. R package version 1.1–20:1–113, http://CRAN.R-project.org/package=lme4
Beckmann C, Biro PA, Martin K (2015) Hierarchical analysis of avian re-nesting behavior: mean, across-individual, and intra-individual responses. Behav Ecol Sociobiol 69:1631–1638
Beier J (1981) Untersuchungen an Drossel- und Teichrohrsänger (Acrocephalus arundinaceus, A. scirpaceus): Bestandsentwicklung, Brutbiologie, Ökologie. J Ornithol 122:209–230
Berger-Tal R, Berger-Tal O, Munro K (2010) Nest desertion by grey fantails during nest building in response to perceived predation risk. J Field Ornithol 81:151–154
Boulton RL, Cassey P, Schipper C, Clarke MF (2003) Nest site selection by yellow-faced honeyeaters Lichenostomus chrysops. J Avian Biol 34:267–274
Campobello D, Sealy SG (2009) Avian brood parasitism in a Mediterranean region: hosts and habitat preferences of common cuckoos Cuculus canorus. Bird Study 56:389–400
Catlin DH, Rosenberg DK (2008) Breeding dispersal and nesting behavior of burrowing owls following experimental nest predation. Am Midl Nat 159:1–7
Chalfoun AD, Martin TE (2010) Facultative nest patch shifts in response to nest predation risk in the brewer’s sparrow: a ‘win-stay, lose-switch’ strategy? Oecologia 163:885–892
Cramp S (ed) (1992) The birds of the Western Palearctic. Warblers, vol. 6. Oxford University Press, Oxford
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
Davies NB, de Brooke ML (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Dröscher L (1988) A study on radio-tracking of the European cuckoo (Cuculus canorus canorus). In: Proceedings of the International Centennial Meeting of the Deutsche Ornithologen-Gesellschaft, Bonn, pp 187–193
Dyrcz A (1981) Breeding ecology of great reed warbler Acrocephalus arundinaceus and reed warbler Acrocephalus scirpaceus at fish-ponds in SW Poland and lakes in NW Switzerland. Acta Ornithol 18:307–334
Fleischer RC (1985) A new technique to identify and assess the dispersion of eggs of individual brood parasites. Behav Ecol Sociobiol 17:91–99
Gärtner K (1981) Das Wegnehmen von Wirtsvogeleiern durch den Kuckuck Cuculus canorus. Ornithol Mitt 33:115–131
Gehringer F (1979) Étude sur le pillage par le coucou, Cuculus canorus, des oeufs de la rousserolle effarvatte. Nos Oiseaux 35:1–16
Geltsch N, Ban M, Hauber ME, Moskát C (2016) When should common cuckoos Cuculus canorus lay their eggs in host nests? Bird Stud 63:46–51
Graham DS (1988) Responses of five host species to cowbird parasitism. Condor 90:588–591
Grégoire A, Cherry MI (2007) Nesting success and within-season breeding dispersal in the orange-breasted sunbird Anthobaphes violacea. Ostrich 78:633–636
Greig-Smith PW (1982) Dispersal between nest-sites by stonechats Saxicola torquata in relation to previous breeding success. Ornis Scand 13:232–238
Guigueno MF, Sealy SG (2010) Clutch abandonment by parasitized yellow warblers: egg burial or nest desertion? Condor 112:399–406
Hanley D, Šulc M, Brennan PLR, Hauber ME, Grim T, Honza M (2016) Dynamic egg color mimicry. Ecol Evol 6:4192–4202
Hansson B, Bensch S, Hasselquist D (2000) The quality and the timing hypotheses evaluated using data on great reed warblers. Oikos 90:575–581
Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E (2002) Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim Behav 64:861–868
Honza M, Šulc M, Jelínek V, Požgayová M, Procházka P (2013) Brood parasites lay eggs matching the appearance of host clutches. Proc R Soc Lond B 281:1471–2954
Hoover JP (2003) Multiple effects of brood parasitism reduce the reproductive success of prothonotary warblers, Protonotaria citrea. Anim Behav 65:923–934
Hoover JP, Yasukawa K, Hauber ME (2006) Spatially and temporally structured avian brood parasitism affects the fitness benefits of hosts’ rejection strategies. Anim Behav 72:881–890
Hosoi SA, Rothstein SI (2000) Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim Behav 59:823–840
Howlett JS, Stutchbury BJM (1997) Within-season dispersal, nest-site modification, and predation in renesting hooded warblers. Wilson Bull 109:643–649
Jackson WM, Rohwer S, Nolan V (1989) Within-season breeding dispersal in prairie warblers and other passerines. Condor 91:233–241
Jelínek V, Procházka P, Požgayová M, Honza M (2014) Common cuckoos Cuculus canorus change their nest-searching strategy according to the number of available host nests. Ibis 156:189–197
Jelínek V, Požgayová M, Honza M, Procházka P (2016) Nest as an extended phenotype signal of female quality in the great reed warbler. J Avian Biol 47:1–10
Koleček J, Jelínek V, Požgayová M, Trnka A, Baslerová P, Honza M, Procházka P (2015) Breeding success and brood parasitism affect return rate and dispersal distances in the great reed warbler. Behav Ecol Sociobiol 69:1845–1853
Korner-Nievergelt F, Robinson R (2015) Methods to analyse ring re-encounter data. R package version 1.3:1–33, https://CRAN.R- project.org/package=birdring
Kus B (2002) Fitness consequences of nest desertion in an endangered host, the least Bell’s vireo. Condor 104:795–802
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 49:1185–1209
Moksnes A, Røskaft E (1989) Adaptations of meadow pipits to parasitism by the common cuckoo. Behav Ecol Sociobiol 24:25–30
Moksnes A, Røskaft E, Hagen LG, Honze M, Mørk C, Olsen PH (2000) Common cuckoo Cuculus canorus and host behaviour at reed warbler Acrocephalus scirpaceus nests. Ibis 142:247–258
Molina-Morales M, Martínez JG, Avilés JM (2012) Factors affecting natal and breeding magpie dispersal in a population parasitized by the great spotted cuckoo. Anim Behav 83:671–680
Moskát C, Honza M (2000) Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography 23:335–341
Moskát C, Szentpéteri J, Barta Z (2002) Adaptations by great reed warblers to brood parasitism: a comparison of populations in sympatry and allopatry with the common cuckoo. Behaviour 139:1313–1329
Moskát C, Hansson B, Barabás L, Bártol I, Karcza Z (2008) Common cuckoo Cuculus canorus parasitism, antiparasite defence and gene flow in closely located populations of great reed warblers Acrocephalus arundinaceus. J Avian Biol 39:663–671
Moskát C, Bán M, Fülöp A, Bereczki J, Hauber ME (2019) Bimodal habitat use in brood parasitic common cuckoos (Cuculus canorus) revealed by GPS telemetry. Auk 136:1–12
Payne RD (2005) The cuckoos. Oxford University Press, Oxford
Pinkowski BC (1977) Breeding adaptations in the eastern bluebird. Condor 79:289–302
Powell LA, Frasch LL (2000) Can nest predation and predator type explain variation in dispersal of adult birds during the breeding season? Behav Ecol 11:437–443
Požgayová M, Procházka P, Honza M (2009) Sex-specific defence behaviour against brood parasitism in a host with female-only incubation. Behav Process 81:34–38
Procházka P, Konvičková-Patzenhauerová H, Požgayová M, Trnka A, Jelínek V, Honza M (2014) Host genotype and age have no effect on rejection of parasitic eggs. Naturwissenschaften 101:417–426
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, https://www.R-project.org/
Røskaft E, Moksnes A, Meilvang D, Bičík V, Jemelíková J, Honza M (2002a) No evidence for recognition errors in Acrocephalus warblers. J Avian Biol 33:31–38
Røskaft E, Moksnes A, Stokke G, Moskát C, Honza M (2002b) The spatial habitat structure of host populations explains the pattern of rejection behavior in hosts and parasitic adaptations in cuckoos. Behav Ecol 13:163–168
Rothstein SI (1990) A model system for coevolution: avian brood parasitism. Annu Rev Ecol Syst 21:481–508
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schulze-Hagen K (1992) Parasitierung und Brutverluste durch Kuckuck (Cuculus canorus) bei Teich- und Sumpfrohrsänger (Acrocephalus scirpaceus, A. palustris) in Mittel- und Westeuropa. J Ornithol 133:237–249
Sedgwick JA (2004) Site fidelity, territory fidelity, and natal philopatry in willow flycatchers (Empidonax traillii). Auk 121:1103–1121
Soler JJ, Møller AP, Soler M (1999) A comparative study of host selection in the European cuckoo Cuculus canorus. Oecologia 118:265–276
Soler M, Ruiz-Raya F, Roncalli G, Ibáñez-Álamo JD (2015) Nest desertion cannot be considered an egg-rejection mechanism in a medium-sized host: an experimental study with the common blackbird Turdus merula. J Avian Biol 46:369–377
Sosnovcová K, Koleček J, Požgayová M, Jelínek V, Šulc M, Steidlová P, Honza M, Procházka P (2018) Timing of natal nests is an important factor affecting return rates of juvenile great reed warblers. J Ornithol 159:183–190
Šulc M, Troscianko J, Štětková G, Hughes AE, Jelínek V, Čapek M, Honza M (2019) Mimicry cannot explain rejection type in a host–brood parasite system. Anim Behav 155:111–118
Welbergen JA, Davies NB (2009) Strategic variation in mobbing as a front line of defense against brood parasitism. Curr Biol 19:235–240
Williams EJ, Boyle WA (2019) Causes and consequences of avian within-season dispersal decisions in a dynamic grassland environment. Anim Behav 155:77–87
Williams HM, Willemoes M, Klaassen RHG, Strandberg R, Thorup K (2016) Common cuckoo home ranges are larger in the breeding season than in the non-breeding season and in regions of sparse forest cover. J Ornithol 157:461–469
Zölei A, Bán M, Moskát C (2015) No change in common cuckoo Cuculus canorus parasitism and great reed warblers’ Acrocephalus arundinaceus egg rejection after seven decades. J Avian Biol 46:570–576
Acknowledgements
We would like to thank Miroslav Čapek, Lucie Halová, Václav Jelínek, Tereza Karasová, Klára Morongová, Radka Piálková, Zuzana Šebelíková, Petra Steidlová, Michal Šulc and Klára Žabková for their help in the field. We are grateful to the managers of the Hodonín Fish Farm for the permission to conduct the fieldwork on their grounds and to two anonymous reviewers for their constructive comments.
Funding
The study was supported by the Czech Science Foundation (grant numbers 17-12262S and 20-00648S) and by the Institutional Research Plan (RVO: 68081766). JK was supported by the Charles University UNCE program (UNCE/SCI/005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research was conducted in compliance with ethical guidelines. This study was carried out with the permission of the regional nature conservation authorities that allowed us to work on wild protected animals (Czech permit numbers 00312/PA/2008/AOPK, JMK20189/2010, JMK 115874/2013). Bird catching and ringing was conducted under licence (numbers 906, 1023, 1050, 1058) and followed the rules issued by the Czech Bird Ringing Centre. The fieldwork adhered to the Animal Care Protocol of the Academy of Sciences of the Czech Republic (numbers 173/2008, 128/2010, 039/2011) and was in compliance with the current Czech Law on the Protection of Animals against Mistreatment (licence numbers CZ 01284, CZ 01272).
Additional information
Communicated by M. Soler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sosnovcová, K., Požgayová, M., Procházka, P. et al. Within-season dispersal does not protect re-nesting great reed warblers (Acrocephalus arundinaceus) from repeated common cuckoo (Cuculus canorus) parasitism. Behav Ecol Sociobiol 74, 69 (2020). https://doi.org/10.1007/s00265-020-02846-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02846-9