Abstract
Rationale
Menthol is a widely used tobacco constituent that has shown to enhance nicotine’s reinforcing effects.
Objective
To determine whether injected menthol also alters nicotine’s stimulus effects, we used a drug discrimination task.
Methods
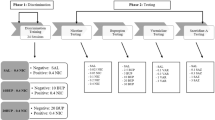
A total of 57 adult Sprague-Dawley rats (28M, 29F) received 20 positive and 20 negative days (intermixed) of discrimination training. On positive days, rats received a group-specific menthol and nicotine injection (VEH + 0.1 NIC, 1 M + 0.1 NIC, 5 M + 0.1 NIC, VEH + 0.4 NIC, 1 M + 0.4 NIC, 5 M + 0.4 NIC; mg/kg) before eight 15-s cue light presentations (conditioned stimulus (CS)), each followed by 4-s sucrose access. On negative days, all rats were injected with vehicle and saline before eight non-reinforced CS presentations. Next, rats underwent generalization testing with 30 dose combinations of menthol and nicotine. The change in drug-mediated anticipatory goal tracking during the CS was calculated as a difference score (CS minus pre-CS responding).
Results
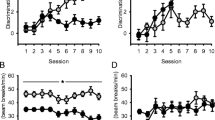
All groups readily acquired drug discrimination. However, difference scores for the 5M + 0.1 NIC group were lower for females. Additionally, females had lower scores for 0.05, 0.1, and 0.4 mg/kg nicotine generalization tests. The lowest nicotine dose discriminable from saline was 0.05 mg/kg for females but 0.025 mg/kg for males. Co-administration with 5 or 10 mg/kg menthol weakened discrimination performance between 0.1 and 0.4 mg/kg and between 0.1 and 0.05 mg/kg nicotine for 0.1 mg/kg nicotine training groups.
Conclusions
Female rats that were trained with 0.1 mg/kg nicotine were more sensitive to menthol’s modulatory effects on nicotine’s stimulus effects. This highlights the importance of taking sex and training dose into account when evaluating the interoceptive stimulus effects of nicotine and menthol.





Similar content being viewed by others
References
Abobo CV, Ma J, Liang D (2012) Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14(7):801–808 10.1093/ntr/ntr287
Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR (2016) Menthol content in U.S. marketed cigarettes. Nicotine Tob Res 18(7):1575–1580 10.1093/ntr/ntv162
Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ et al (2015) Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One 10(9):e0137070 10.1371/journal.pone.0137070
Benowitz NL, Herrera B, Jacob P (2004) Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther 310(3):1208–1215 10.1124/jpet.104.066902
Besheer J, Palmatier MI, Metschke DM, Bevins RA (2004) Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology 172(1):108–117 10.1007/s00213-003-1621-9
Bevins RA, Charntikov S (2015) We know very little about the subjective effects of drugs in females. ACS Chem Neurosci 6:359–361 10.1021/acschemneuro.5b00018
Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM (2006) Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology 184(3-4):470–481 10.1007/s00213-005-0079-3
Biswas L, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T et al (2016) Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology 233(18):3417–3427 10.1007/s00213-016-4391-x
Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA (2017) The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology 113(Pt A):354–366 10.1016/j.neuropharm.2016.10.014
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KP (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180(2):258–266. 10.1007/s00213-005-2152-3
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Retrieved from https://www.R-project.org/
Cubbin C, Soobader MJ, Leclere FB (2010) The intersection of gender and race/ethnicity in smoking behaviors among menthol and non-menthol smokers in the United States. Addiction 105(SUPPL.1):32–38 10.1111/j.1360-0443.2010.03191.x
DeVito EE, Valentine GW, Herman AI, Jensen KP, Sofuoglu M (2016) Effect of menthol-preferring status on response to intravenous nicotine. Tob Regul Sci 2:317–328. https://doi.org/10.18001/TRS.2.4.4
Donny E, Caggiula A, Mielke M et al (1998) Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology 136:83–90 10.1007/s002130050542
Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology 151(4):392–405 10.1007/s002130000497
Family Smoking Prevention and Tobacco Control Act (2009) H.R. 1256. 111th Cong., 1st Sess., 155, E859–859
Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, Jordt SE (2016) Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tob Control 25:ii50–ii54 10.1136/tobaccocontrol-2016-053209
Farco JA, Grundmann O (2013) Menthol - pharmacology of an important naturally medicinal “Cool”. Mini-Rev Med Chem 13(1):124–131. https://doi.org/10.2174/138955713804484686
Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS (2010) Do smokers of menthol cigarettes find it harder to quit smoking? Nicotine Tob Res 12(Suppl 2):S102–S109 10.1093/ntr/ntq166
Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, Vallone DM, Abrams DB (2013) Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control 24:28–37 10.1136/tobaccocontrol-2013-051159
Hans M, Wilhelm M, Swandulla D (2012) Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses 37:463–469 10.1093/chemse/bjr128
Harrison E, Biswas L, Avusula R, Zhang M, Gong Y, Liu X (2017) Effects of menthol and its interaction with nicotine-conditioned cue on nicotine-seeking behavior in rats. Psychopharmacology 234:3443–3453 10.1007/s00213-017-4736-0
Henderson BJ, Wall TR, Henley BM, Kim CH, Nichols WA, Moaddel R, Xiao C, Lester HA (2016) Menthol alone upregulates midbrain nAChRs, alters nAChR subtype stoichiometry, alters dopamine neuron firing frequency, and prevents nicotine reward. J Neurosci 36(10):2957–2974 10.1523/JNEUROSCI.4194-15.2016
Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA (2017) Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42(12):2285–2291 10.1038/npp.2017.72
Huynh YW, Raimondi A, Schuster C, Finkner A, Selleck C, Bevins RA (2019) Investigating the interoceptive stimulus effects of injected menthol in rats. Exp Clin Psychopharmacol 28:19–25. https://doi.org/10.1037/pha0000295
Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016) Intravenous nicotine self-administration in smokers: dose-response function and sex differences. Neuropsychopharmacology 41(8):2034–2040 10.1038/npp.2015.373
Kasza KA, Hyland AJ, Bansal-Travers M, Vogl LM, Chen J, Evans SE, Fong GT, Cummings KM, O’Connor RJ (2014) Switching between menthol and nonmenthol cigarettes: findings from the U.S. Cohort of the International Tobacco Control Four Country Survey. Nicotine Tob Res 16(9):1255–1265 10.1093/ntr/ntu098
Kuiper NM, Gammon D, Loomis B, Falvey K, Wang TW, King BA, Rogers T (2017) Trends in sales of flavored and menthol tobacco products in the United States during 2011–2015. Nicotine Tob Res 20(6):698–706 10.1093/ntr/ntx123
Lenth R (2019) Emmeans: estimated marginal means, aka least-squares means. Retrieved from https://CRAN.R-project.org/package=emmeans
McClernon FJ, Kozink RV, Rose JE (2008) Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 33:2148–2157 10.1038/sj.npp.1301618
Murray JE, Li C, Palmatier MI, Bevins RA (2007) The interoceptive Pavlovian stimulus effects of caffeine. Pharmacol Biochem Behav 86(4):838–846 10.1016/j.pbb.2007.03.013
Nesil T, Narmeen S, Bakhti-Suroosh A, Lynch WJ (2018) Effect of menthol on nicotine intake and relapse vulnerability in a rat model of concurrent intravenous menthol/nicotine self-administration. Psychopharmacology 236(4):1219–1232 10.1007/s00213-018-5128-9
Palmatier MI, Bevins RA (2008) Occasion-setting by drug states: functional equivalence following similar training history. Behav Brain Res 195(2):260–270 10.1016/j.bbr.2008.09.009
Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA (2004) Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol 15(3):183–194 10.1097/01.fbp.0000132915.11693.8e
Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA (2005) Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology 30(4):731–741 10.1038/sj.npp.1300629
Perkins KA, Kunkle N, Karelitz JL (2017) Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs. non-menthol smokers. Psychopharmacology 234(8):1255–1265 10.1007/s00213-017-4563-3
Perkins KA, Karelitz JL, Kunkle N (2018) Sex differences in subjective responses to moderate versus very low nicotine content cigarettes. Nicotine Tob Res 20(10):1258–1264 10.1093/ntr/ntx205
Singmann H, Bolker B, Westfall J, Aust F (2019) Afex: analysis of factorial experiments. Retrieved from https://CRAN.R-project.org/package=afex
Smith SS, Fiore MC, Baker TB (2014) Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction 109(2):2107–2117 10.1111/add.12661
Smith PH, Akpara E, Haq R, El-Miniawi M, Thompson AB (2017) Gender and menthol cigarette use in the United States: a systematic review of the recent literature (2011 - May 2017). Curr Addict Rep 4(4):431–438 10.1007/s40429-017-0175-6
Sofuoglu M, Mooney M (2009) Subjective responses to intravenous nicotine: greater sensitivity in women than in men. Exp Clin Psychopharmacol 17(2):63–69 10.1037/a0015297
Stolerman IP, Garcha HS, Pratt JA, Kumar R (1984) Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology 84(3):413. 10.1007/BF00555223–419
Thompson MF, Poirier GL, Dávila-García MI, Huang W, Tam K, Robidoux M, Dubuke ML, Shaffer SA, Colon-Perez L, Febo M, DiFranza J, King JA (2017) Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. J Psychopharmacol 32(3):332–343 10.1177/0269881117719265
Tukey J (1949) Comparing individual means in the analysis of variance. Biometrics 5(2):99–114. https://doi.org/10.2307/3001913
U.S. Department of Health and Human Services (2014) The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta. Retrieved from https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf
Wang T, Wang B, Chen H (2014) Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci 8:1–12 10.3389/fnbeh.2014.00437
Wickham H (2016) ggplot2: elegant graphics for data analysis. Retrieved from https://cran.r-project.org/web/packages/ggplot2/index.html
Wickham RJ, Nunes EJ, Hughley S, Silva P, Walton SN, Park J, Addy NA (2018) Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology 128:33–42 10.1016/j.neuropharm.2017.09.029
World Health Organization (2011) WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/44616/9789240687813_eng.pdf;jsessionid=296AA6EC8A23C7A0F83EE98209115B48?sequence=1
Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF (2015) Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med 48(3):326–333 10.1016/j.amepre.2014.10.012
Zhang M, Harrison E, Biswas L, Tran T, Liu X (2018) Menthol facilitates dopamine-releasing effect of nicotine in rat nucleus accumbens. Pharmacol Biochem Behav 175(1):47–52 10.1016/j.pbb.2018.09.004
Acknowledgments
We thank Ashlyn Saeger and Allissa Flynn for the help with conducting daily experimental sessions.
Funding
This study was supported by a grant from the National Institutes of Health, R01-DA034389 and R01-DA046109. The funding sources had no other role than financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huynh, Y.W., Raimondi, A., Finkner, A. et al. Menthol blunts the interoceptive discriminative stimulus effects of nicotine in female but not male rats. Psychopharmacology 237, 2395–2404 (2020). https://doi.org/10.1007/s00213-020-05542-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05542-8