Abstract
Background
The use of immunosuppressive therapy for IgA nephropathy patients with renal insufficiency and severe proteinuria is controversial.
Methods
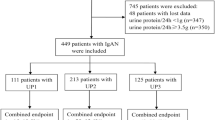
This was a monocentric retrospective study. We reviewed 132 consecutive IgA nephropathy (IgAN) patients with stage 3 or 4 chronic kidney disease and proteinuria ≥ 1.0 g/d who received uncontrolled supportive care (n = 41), corticosteroids (CS) (n = 22) or low-dose CS combined with oral cyclophosphamide (CTX) (n = 69) between January 2008 and December 2016. The combined endpoint was defined as either a ≥ 50% reduction in eGFR or ESRD.
Results
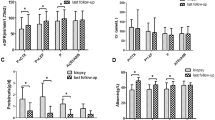
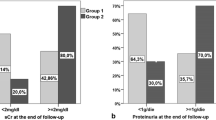
All patients were followed for a medial of 33.2 months, and 67 (50.8%) patients experienced the combined endpoint. The rate of renal function decline was − 4.5 (− 12.6, − 0.1) ml/min/1.73 m2 per year. In multivariate Cox regression analyses, immunosuppressive therapy (HR = 0.349, 95% CI 0.194–0.629, P < 0.001) was associated with reduced risk of combined events after adjusting for age, sex, MAP, proteinuria, eGFR, mesangial hypercellularity score > 0.5 (M1), endocapillary hypercellularity present (E1), segmental glomerulosclerosis present (S1), tubular atrophy/interstitial fibrosis > 25% (T1–2), crescents present (C1–2), and RAAS blockers. Immunosuppressive therapy was also analyzed as a categorical variable, and multivariate Cox analyses showed that CS did not reduce the risk of combined events, whereas CS + CTX significantly reduced the risk of combined events. In the matched cohort, the CS + CTX group had a significantly lower reduction in TP-A [1.2 (0.6, 2.3) g/d verse 1.8 (1.2, 2.5), P = 0.023] and a better renal survival rate (39.4% verse 66.7%, P = 0.026) than the uncontrolled supportive care group. The number of hospitalizations required for infection was similar in the three study groups. Other adverse events did not differ significantly among the three groups.
Conclusion
Low-dose CS combined with oral CTX treatment is possibly more effective than uncontrolled supportive care for IgAN patients with reduced renal function. The results need to be further confirmed by randomized controlled studies.



Similar content being viewed by others
References
Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Investig 119(6):1668–1677. https://doi.org/10.1172/JCI38468
Glassock RJ (2009) Analyzing antibody activity in IgA nephropathy. J Clin Investig 119(6):1450–1452. https://doi.org/10.1172/jci39189
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group (2012) KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2:139–274
Pozzi C, Sarcina C, Ferrario F (2016) Treatment of IgA nephropathy with renal insufficiency. J Nephrol 29(4):551–558. https://doi.org/10.1007/s40620-015-0257-2
Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F (1999) Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 353(9156):883–887. https://doi.org/10.1016/s0140-6736(98)03563-6
Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, Vogt B, De Cristofaro V, Allegri L, Cirami L, Procaccini AD, Locatelli F (2010) Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol JASN 21(10):1783–1790. https://doi.org/10.1681/ASN.2010010117
D'Amico G, Ragni A, Gandini E, Fellin G (1993) Typical and atypical natural history of IgA nephropathy in adult patients. Contrib Nephrol 104:6–13. https://doi.org/10.1159/000422389
Coppo R (2017) Clinical and histological risk factors for progression of IgA nephropathy: an update in children, young and adult patients. J Nephrol 30(3):339–346. https://doi.org/10.1007/s40620-016-0360-z
Coppo R, Robert T (2020) IgA nephropathy in children and in adults: two separate entities or the same disease? J Nephrol. https://doi.org/10.1007/s40620-020-00725-0
Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, Ito K, Iitaka K, Koitabashi Y, Yamaoka K, Nakagawa K, Nakamura H, Matsuyama S, Seino Y, Takeda N, Hattori S, Ninomiya M (1999) A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy: the Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol JASN 10(1):101–109
Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW (2006) Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354(2):131–140. https://doi.org/10.1056/NEJMoa053107
Ballardie FW, Roberts IS (2002) Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol JASN 13(1):142–148
Shin JH, Lee JE, Park JH, Lim S, Jang HR, Kwon GY, Huh W, Jung SH, Kim YG, Oh HY, Kim DJ (2016) The effects of cytotoxic therapy in progressive IgA nephropathy. Ann Med 48(3):171–181. https://doi.org/10.3109/07853890.2016.1153805
Fang J, Li W, Li D, Tan Z (2014) Baseline proteinuria, urinary osmotic pressure, and renal function as positive predictors of corticosteroids plus cyclophosphamide treatment efficacy in IgA nephropathy. Chin Med J 127(9):1710–1714
Oshima S, Kawamura O (2008) Long-term follow-up of patients with IgA nephropathy treated with prednisolone and cyclophosphamide therapy. Clin Exp Nephrol 12(4):264–269. https://doi.org/10.1007/s10157-008-0045-6
Rasic S, Uncanin S, Aganovic K, Rasic I, Dzemidzic J, Muslimovic A (2008) Treatment of IgA nephropathy of adults presented by nephrotic syndrome. Bosn J Basic Med Sci 8(3):230–233. https://doi.org/10.17305/bjbms.2008.2923
Mitsuiki K, Harada A, Okura T, Higaki J (2007) Histologically advanced IgA nephropathy treated successfully with prednisolone and cyclophosphamide. Clin Exp Nephrol 11(4):297–303. https://doi.org/10.1007/s10157-007-0497-0
Tsuruya K, Harada A, Hirakata H, Mitsuiki K, Johko T, Kondoh H, Takechi S, Fujishima M (2000) Combination therapy using prednisolone and cyclophosphamide slows the progression of moderately advanced IgA nephropathy. Clin Nephrol 53(1):1–9
Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A, Group VsotE-EIW (2014) Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86(4):828–836. https://doi.org/10.1038/ki.2014.63
Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, Pecchini P, Rustichelli R, Finocchiaro P, Del Vecchio L, Locatelli F (2013) IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. J Nephrol 26(1):86–93. https://doi.org/10.5301/jn.5000110
Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J, Investigators ST-I (2015) Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373(23):2225–2236. https://doi.org/10.1056/NEJMoa1415463
Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J, Ig ANCWGotIINN, the Renal Pathology S, Conference P (2017) Oxford classification of IgA NEPHROPATHY 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 91(5):1014–1021. https://doi.org/10.1016/j.kint.2017.02.003
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Scholl U, Wastl U, Risler T, Braun N, Grabensee B, Heering P, Schollmeyer P, Zauner I, Stein G, Funfstuck R, Keller F (1999) The "point of no return" and the rate of progression in the natural history of IgA nephritis. Clin Nephrol 52(5):285–292
Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V, Group TS (2017) Effect of oral methylprednisolone on clinical outcomes in patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 318(5):432–442. https://doi.org/10.1001/jama.2017.9362
Hotta O, Furuta T, Chiba S, Tomioka S, Taguma Y (2002) Regression of IgA nephropathy: a repeat biopsy study. Am J Kidney Dis 39(3):493–502. https://doi.org/10.1053/ajkd.2002.31399
Pozzi C, Ferrario F, Visciano B, Del Vecchio L (2012) Corticosteroids in patients with IgA nephropathy and severe chronic renal damage. Case Rep Nephrol 2012:180691. https://doi.org/10.1155/2012/180691
Ponticelli C, Glassock RJ (2019) Prevention of complications from use of conventional immunosuppressants: a critical review. J Nephrol 32(6):851–870. https://doi.org/10.1007/s40620-019-00602-5
Julian BA, Barker C (1993) Alternate-day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol 104:198–206
Acknowledgements
We thank the staff of the department of Nephrology, Xijing Hospital. This study was partially supported by grants from the National Natural Science Foundation of China (81670655), Key research and development plan of Shaanxi province, China (No. 2017ZDXM-SF-045). The discipline boosting program of the Xijing Hospital of the Fourth Military Medical University (XJZT18Z15). All the authors declared no competing interests.
Funding
This study was partially supported by grants from the National Natural Science Foundation of China (81670655), Key research and development plan of Shaanxi province, China (No. 2017ZDXM-SF-045). The discipline boosting program of the Xijing Hospital of the Fourth Military Medical University (XJZT18Z15).
Author information
Authors and Affiliations
Contributions
FM and XXY designed the study, acquired data, interpreted data, drafted and revised the manuscript. MLZ, MB, LJZ and LL analyzed and interpreted data, drafted and revised the manuscript. RJD, CML and RL acquired data, interpreted data and revised the manuscript. SRS designed the study, revised the manuscript, assumed responsibility for the integrity of the data and supervised the research group. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest need to be declared.
Ethical statement
The study was approved by the Ethics Committee of Xijing Hospital. This study waived the requirement for written informed consent due to the retrospective nature of this study. This study protocol was performed in accordance with the 1964 Declaration of Helsinki as well as the amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, F., Yang, X., Zhou, M. et al. Treatment for IgA nephropathy with stage 3 or 4 chronic kidney disease: low-dose corticosteroids combined with oral cyclophosphamide. J Nephrol 33, 1241–1250 (2020). https://doi.org/10.1007/s40620-020-00752-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00752-x