Abstract
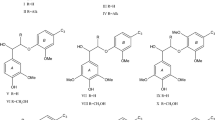
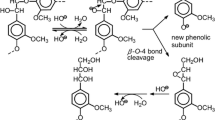
Lignin valorization is strongly dependent on a right strategy for converting lignin into value-added products. Conversion of lignin into monomeric degradation products is one of the important avenues. In this study, chemical mechanisms and monomeric product compositions in hydrolysis (acidic and alkaline), hydrogenolysis, catalytic oxidation, electrochemical oxidation and reduction, photochemical and enzymatic degradation of native and technical lignins, and lignin model compounds are comparatively analyzed. The effect of the structure of a phenylpropane unit in lignin on the chemical reactivity of α-O-4 and β-O-4 bonds in cleavage reactions is also described. Published experimental data suggest some form of activation to be a necessary prerequisite for the splitting of a β-O-4 bond in all chemical reactions under consideration, the nature of which is dependent on the reagents and reaction conditions. Thus, in catalytic oxidation processes, a benzyl hydroxyl group is converted into carbonyl group at the first stage. Chemical transformation involving the α-position in a phenylpropane unit is a usual trigger of further lignin depolymerization. The yield of monomeric products of hydrogenolysis of native lignin is close to the theoretical one, reaching 23% in softwood and 51% in hardwood; in alkaline hydrolysis as well as oxygen and nitrobenzene oxidation of native lignin, the trend is the same that is explained in this study. On the other hand, the yield of monomeric products from isolated samples and technical lignins is much lower. A loss of arylether bonds in the process of lignin separation from wood and in wood pulping explains this difference.




























Similar content being viewed by others
References
Abdelaziz OY, Brink DP, Prothmannc J, Ravi K, Sun M, García-Hidalgo J, Sandahl M, Hulteberg CP, Turner C, Lidén G, Gorwa-Grauslund MF (2016) Biological valorization of low molecular weight lignin. Biotechnol Adv 34:1318–1346
Agarwal A, Rana M, Jeong Hun P (2018) Advancement in technologies for the depolymerization of lignin. Fuel Proc Technol 181:115–132
Azadi P, Inderwildi OR, Farnood R, King DA (2013) Liquid fuels, hydrogen and chemicals from lignin: a critical review. Renew Sustain Energy Rev 21:506–523
Beckham GT, Ed. (2018) Lignin valorization: emerging approaches. Energy Envir Series No. 19, The Royal Soc of Chem, Croydon
Behling R, Valange S, Chatel G (2016) Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: What results? What limitations? What trends? Green Chem 18:1839–1854
Berlin M, Balakshin M (2014) Industrial lignins, analysis, properties and applications. Bioenergy Res Adv Appl Chapter 18:315–336
Bosque I, Magallanes J, Rigoulet M, Kärkäs MD, Stephenson CRJ (2017) Redox catalysis facilitates lignin depolymerization. ACS Cent Sci 3:621–628
Bruijnincx P, Weckhuysen B, Gruter G-J, Westenbroek A (2016) Lignin valorisation. The importance of a full value chain approach. Utrecht University, Platform Agro-Paper-Chemistry 1–22
Carlton WD, Reeve DW (eds) (1996) Pulp bleaching Principles and practice. Tappi Press, Atlanta
Capanema EA, Balakshin MY, Kadla JF (2004) A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J Agr Food Chem 52:1850–1860
Chatel G, Rogers RD (2014) Oxidation of lignin using ionic liquids—an innovative strategy to produce renewable chemicals. ACS Sustainable Chem Eng 2–3:322–339
Chen J, Lu F, Si X, Nie X, Chen J, Lu R, Xu J (2016) High yield production of natural phenolic alcohols from woody biomass using a nickel-based catalyst. Chemsuschem 9:3353–3360
Chen J, Liu W, Song Z, Wang H, Xie Y (2018) Photocatalytic degradation of β-O-4 lignin model compound by In2S3 nanoparticles under visible light irradiation. Bioenergy Res 11:166–173
Chio C, Sain M, Qin W (2019) Lignin utilization: A review of lignin depolymerization from various aspects. Renew Sustain Energy Rev 107:232–249
Collinson SR, Thielemans W (2010) The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord Chem Rev 254:1854–1870
Cox BJ, Jia S, Zhang ZC, Ekerdt JG (2011) Catalytic degradation of lignin model compounds in acidic imidazolium based ionic liquids: hammett acidity and anion effects. Polym Degrad Stab 96:426–431
Cox BJ, Ekerdt JG (2012) Depolymerization of oak wood lignin under mild conditions using the acidic ionic liquid 1-H-3-methylimidazolium chloride as both solvent and catalyst. Biores Technol 118:584–588
Creary X, Willis ED, Gagnon M (2005) Carbocation-forming reactions in ionic liquids. J Am Chem Soc 127:18114–18120
Crestini C, Caponi MC, Argyropoulos DS, Saladino R (2006) Immobilized methyltrioxorhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorg Med Chem 14:5292–5302
Davis KM, Rover M, Brown RC, Bai X, Wen Z, Jarboe LR (2016) Recovery and utilization of lignin monomers as part of the biorefinery approach. Energies 9:808–836
Deuss PJ, Barta K (2016) From models to lignin: transition metal catalysis for selective bond cleavage reactions. Coord Chem Rev 306:510–532
de Gonzaloa G, Colpab DI, Habibb MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
Evstigneyev EI (2012) Path of the Fiber The Effect of Wood Structure on Pulp and Paper Technologies. Chimizdat, St.-Petersburg (in Russian)
Evstigneyev EI (2014) Electrochemical reactions of lignin: a review. Khimija Rastitelnogo Syr’ja Chem Plant Resour 3:5–42 (in Russian)
Evstigneyev EI (2018) Selective depolymerization of lignin: assessment of yields of monomeric products. J Wood Chem Technol 38:409–415
Evstigneyev EI, Shevchenko SM (2019) Structure, chemical reactivity and solubility of lignin: a fresh look. Wood Sci Technol 53:7–47
Evstigneyev E, Shevchenko S, Maiyorova H, Platonov A (2004) Polarographically active structural fragments of lignin II Dimeric model compounds and lignins. J Wood Chem Technol 24:263–278
Evstigneyev EI, Yuzikhin OS, Gurinov AA, Ivanov AYu, Artamonova TO, Khodorkovskiy MA, Bessonova EA, Vasilyev AV (2016) Study of structure of industrial acid hydrolysis lignin, oxidizedin the H2O2–H2SO4 system. J Wood Chem Technol 36:259–269
Evstigneyev E, Kalugina AV, Ivanov AYu, Vasilyev AV (2017) Contents of α-O-4 and β-O-4 bonds in native lignin and isolated lignin preparations. J Wood Chem Technol 37:294–306
Evtuguin DV, Daniel AID, Silvestre AJD, Amado FML, Neto CP (2000) Lignin aerobic oxidation promoted bymolybdovanadophosphate polyanion [PMo7V5O40](8-). Study on the oxidative cleavage of beta-O-4 aryl ether structures using model compounds. J Mol Catal A Chem 154:217–224
Feghali E, Carrot G, Thueґry P, Genre C, Cantat T (2015) Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. Energy Environ Sci 8:2734–2743
Fengel D, Wegener G (1989) Wood: Chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin, NY
Galkin MV, Samec JSM (2014) Selective route to 2-propenyl aryls directly from wood by a tandem organosolv and palladium-catalysed transfer hydrogenolysis. Chemsuschem 7:2154–2158
Galkin MV, Samec JSM (2016) Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. Chemsuschem 9:1544–1558
Gellerstedt G, Henriksson G (2008) In: Monomers, Polymers and Composites from Renewable Resources. In: Belgacem MN, Gandini A (eds) Amsterdam 201–224
Gierer J (1997) Formation and involvement of superoxide (O2•/HO2•) and hydroxyl (HO•) radicals in TCF bleaching processes: a review. Holzforschung 51:34–46
Gierer J, Lindeberg O (1980) Reaction of lignin during sulfate pulping. Part XIX. Isolation and identification of new dimmers from a spent sulfate liquor. Acta Chem Scand 34(3):161–170
Gierer J, Ljunggren S (1983) Comparative studies of the participation of different neighboring groups in the alkaline cleavage of β-aryl ether bonds in lignin. Svensk Papperstidn 86(9):R100–R106
Holladay JE, White JF, Bozell JJ, Johnson D (2007) Top value-added chemicals from biomass. Vol. II: Results of screening for potential candidates from biorefinery lignin. U.S. Department of Energy, PNNL-16983: 1–79
Huang X, Ouyang X, Hendriks B, Gonzalez OMM, Zhu J, Koranyi, Boot M, EJM, Hensen (2017) Selective production of mono-aromatics from lignocellulose over Pd/C catalyst: on the influence of acid co-catalysts. Faraday Discuss 202:141–156TI
Jia S, Cox BJ, Guo X, Zhang ZC, Ekerdt JG (2010a) Decomposition of a phenolic lignin model compound over organic N bases in an ionic liquid. Holzforschung 64:577–580
Jia S, Cox BJ, Guo X, Zhang ZC, Ekerdt JG (2010b) Cleaving the β-O-4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: an optional strategy for the degradation of lignin. Chemsuschem 3:1078–1084
Jia SY, Cox BJ, Guo XW, Zhang ZC, Ekerdt JG (2011) Hydrolytic cleavage of beta-O-4 ether bonds of lignin model compounds in an ionic liquid with metal chlorides. Ind Eng Chem Res 50:849–855
Kärkäs MD, Matsuura BS, Monos TM, Magallanes G, Stephenson CRJ (2016) Transition-metal catalyzed valorization of lignin: the key to a sustainable carbon-neutral future. Org Biomol Chem 14:1853–1914
Klein I, Marcum C, Kenttämaa H, Abu-Omar MM (2016) Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem 18:2399–2405
Kleinert M, Barth T (2008) Towards a lignin-cellulosic biorefinery: direct one-step conversion of lignin to hydrogen-enriched biofuel. Energy Fuels 22:1371–1379
Kondo R, McCarthy JL (1985) Incremental delignification of hemlock wood and characterization of lignin products. Holzforschung 39(4):231–234
Kondo R, Sarkanen KV (1984a) Kinetics of lignin and hemicellulose dissolution during the initial stage alkaline pulping. Holzforschung 38:31–36
Kondo R, Sarkanen KV (1984b) Formation and reaction of coniferyl alcohol during alkaline pulping. J Wood Chem Technol 4:303–311
Konnerth H, Zhang J, Ma D, Prechtl MHG, Yan N (2015) Base promoted hydrogenolysis of lignin model compounds and organosolv lignin over metal catalysts in water. Chem Eng Sci 123:155–163
Lahive CW, Deuss PJ, Lancefield CS, Sun Z, Cordes DB, Young CM, Tran F, Slawin AMZ, de Vries JG, Kamer PCJ, Westwood NJ, Barta KJ (2016) Advanced model compounds for understanding acid-catalyzed lignin depolymerization: identification of renewable aromatics and a lignin-derived solvent. J Am Chem Soc 138:8900–8911
Lancefield CS, Ojo OS, Tran F, Westwood NJ (2015) Isolation of functionalized phenolic monomers through selective oxidation and C-O bond cleavage of the β-O-4 linkages in lignin. Angew Chem Int Ed 54:258–262
Li B, Filpponen I, Argyropoulos DS (2010) Acidolysis of wood in ionic liquids. Ind Eng Chem Res 49:3126–3136
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Liu Y, Chen L, Wang T, Zhang Q, Wang C, Yan J, Ma L (2015) One-pot catalytic conversion of raw lignocellulosic biomass into gasoline alkanes and chemicals over LiTaMoO6 and Ru/C in aqueous phosphoric acid. ACS Sustain Chem Eng 3:1745–1755
Lu F, Ralph J (1997) DFRC method for lignin analysis. 1. New method for β-aryl ether cleavage: lignin model studies. J Agric Food Chem 45:4655–4660
Lu F, Ralph J (1998a) DFRC method for lignin analysis. 2. Monomers from isolated lignins. J Agric Food Chem 46:547–552
Lu F, Ralph J (1998b) b) DFRC method for lignin analysis. 3. NMR studies J Wood Chem 18:219–233
Lundquist K (1973) Formation of low molecular weight phenol from “milled wood lignin” during sulphate and soda cooking. Svensk Papperstidn 76:704–710
Lundquist K (1976) Low-molecular weight lignin hydrolysis product. Appl Polym Sympos 28:1393–1407
Lundquist K (1992) Acidolysis. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Berlin, pp 289–300
Luo J, Melissa P, Zhao W, Wang Z, Zhu Y (2016a) Selective lignin oxidation towards vanillin in phenol media. Chem Select Comm 1:4596–4601
Luo N, Wang M, Li H, Zhang J, Liu H, Wang F (2016b) Photocatalytic oxidation–hydrogenolysis of lignin β-O-4 models via a dual light wavelength switching strategy. ACS Catal 6:7716–7721
Luo J, Zhang X, Lu J, Zhang J (2017a) Fine tuning the redox potentials of carbazolic porous organic frameworks for visible-light photoredox catalytic degradation of lignin β-O-4 models. ACS Catal 7:5062–5070
Luo N, Wang M, Li H, Zhang J, Hou T, Chen H, Zhang X, Lu J, Wang F (2017b) Visible-light-driven self-hydrogen transfer hydrogenolysis of lignin models and extracts into phenolic products. ACS Catal 7:4571–4580
Ma R, Xu Y, Zhang X (2015) Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. Chemsuschem 8:24–51
Nguyen JD, Matsuura BS, Stephenson CRJ (2014) A photochemical strategy for lignin degradation at room temperature. J Am Chem Soc 136:1218–1221
Niemela K (1990) Low-molecular-weight organic compounds in birch kraft black liquor. Ann Acad Sci Fennica Series A II Chemica 229:1–142
Nahum LS (1965) Delignification of wood by hydrogenation in presence of dicobaltoctacarbonyl. Ind Eng Chem Prod Res Devel 4(2):71–74
Parsell T, Yohe S, Degenstein J, Jarrell T, Klein I, Gencer E, Abu-Omar MM (2015) A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem 17:1492–1499
Peng J, Lu F, Ralph J (1998) The DFRC method for lignin analysis. 4. Lignin isolated from DFRC-degraded loblolly pine wood. J Agric Food Chem 46:553–560
Pepper JM, Lee YW (1969) Lignin and related compounds. I. A comparative study of catalysts for lignin hydrogenolysis. Can J Chem 47:723–727
Pepper JM, Steck W (1963) The effect of time and temperature on the hydrogenation of aspen lignin. Can J Chem 41:2867–2875
Rabinovich ML (2010) Wood hydrolysis industry in the Soviet Union and Russia: A mini-review. Cellulose Chem Technol l44(4–6):173–186
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis M, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843–1–1246910
Rahimi A, Azarpira A, Kim H, Ralph J, Stahl SS (2013) Chemoselective metal-free aerobic alcohol oxidation in lignin. J Am Chem Soc 135:6415–6418
Rahimi A, Ulbrich A, Coon JJ, Stahl SS (2014) Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515:249–252
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PCA, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed 55:8164–8215
Rolando C, Monties B, Lapierre C (1992) Thioacidolysis. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Berlin, pp 334–349
Sarkanen KV, Ludwig CH (eds) (1971) Lignins: occurrence, formation, and reactions. Wiley, New York
Sato Y, Moriuchi H, Hishiyama S, Otsuka OK, Kasai D, Nakamura M, Ohara S, Katayama Y, Fukuda M, Masai E (2009) Identification of three alcohol dehydrogenase genes involved in the stereospecific catabolism ofarylglycerol-β-aryl ether by Sphingobium sp. strain SYK-6. Appl Environ Microbiol 75:5195–5201
Sannami Y, Kamitakahara H, Takano T (2016) TEMPO-mediated electro-oxidation reactions of non-phenolic β-O-4-type lignin model compounds. Holzforschung 71:109–117
Schutyser W, Renders T, Van den Bosch S, Koelewijn S-F, Beckhamb GT, Sels BF (2018) Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem Soc Rev 47(3):852–908
Sergeev AG, Hartwig JF (2011) Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 332(6028):439–443
Shao Y, Xia Q, Dong L, Liu X, Han X, Parker SF, Cheng Y, Daemen LL, Ramirez-Cuesta AJ, Yang S (2017) Selective production of arenes via direct lignin upgrading over a Niobium-based catalyst. Nature Commun 8:16104
Shiraishi T, Takano T, Kamitakahara H, Nakatsubo F (2012a) Studies on electrooxidation of lignin and lignin model compounds. Part 1: Direct electrooxidation of non-phenolic lignin model compounds. Holzforschung 66:303–309
Shiraishi T, Takano T, Kamitakahara H, Nakatsubo F (2012b) Studies on electrooxidation of lignin and lignin model compounds. Part 2: N-Hydroxyphthalimide(NHPI)-mediated indirect electrooxidation of non-phenolic lignin model compounds. Holzforschung 66:311–315
Shorygina NN, Reznikov VM, Yelkin VV (1976) Chemical Reactivity of Lignin. Nauka, Moscow (in Russian)
Shuai L, Amiri MT, Questell-Santiago YM, Héroguel F, Li Y, Kim H, Meilan R, Chapple C, Ralph J, Luterbacher JS (2016) Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerisation. Science 354(6310):329–333
Song Q, Wang F, Cai J, Wang Y, Zhang J, Yu W, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation−hydrogenolysis process. Energy Environ Sci 6:994–1007
Song Y, Mobley JK, Motagamwala AH, Isaacs M, Dumesic JA, Ralph J, Lee AF, Wilsong K, Crocker M (2018) Gold-catalyzed conversion of lignin to low molecular weight aromatics. Chem Sci 9:8127–8133
Sturgeon MR, Kim S, Lawrence K, Paton RS, Chmely SC, Nimlos M, Foust TD, Beckham GT (2014) A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkage: implication for lignin depolymerization in acidic environments. ACS Sustainable Chem Eng 2:472–485
Sun Z, Fridrich B, de Santi A, Elangovan S, Barta K (2018) Bright side of lignin depolymerization: toward new platform chemicals. Chem Rev 118:614–678
Tarabanko VE, Hendogina YV, Petuhov DV, Pervishina EP (2000) On the role of retro-aldol reaction in the process of ligninoxidation into vanillin. Kinetics of the vanillideneacetone cleavage in alkaline media. React Kinet Catal Lett 69:361–368
Tarabanko VE, Petukhov DV, Selyutin GE (2004) New mechanism for the catalytic oxidation of lignin to vanillin. Kinet Catal 45:569–577
Tarabanko VE, Tarabanko N (2017) Catalytic oxidation of lignins into the aromatic aldehydes: general process trends and development prospects. Int J Mol Sci 18:2421–2450
Thevenot M, Dignac M-F, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211
Toledano A, Serrano L, Balu AM, Luque R, Pineda A, Labidi J (2013) Fractionation of organosolv lignin from olive tree clippings and its valorization to simple phenolic compounds. Chemsuschem 6:529–536
Torr KM, van de Pas DJ, Cazeils E, Suckling ID (2011) Mild hydrogenolysis of in-situ and isolated Pinus Radiata lignins. Biores Technol 102:7608–7611
Van den Bosch S, Schutyser W, Vanholme R, Driessen T, Koelewijn S-F, Renders T, De Meester B, Huijgen WJJ, Dehaen W, Courtin CM (2015a) Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ Sci 8:1748–1763
Van den Bosch S, Schutyser W, Koelewijn S-F, Renders T, Courtin CM, Sels BF (2015b) Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem Commun 51:13158–13161
Vardon DR, Franden MA, Christopher W, Johnson CW, Karp EM, Michael T, Guarnieri MT, Linger JG, Salm MJ, Timothy J, Strathmann TJ, Beckham GT (2015) Adipic acid production from lignin. Energy Environ Sci 8:617–628
Vardon DR, Rorrer NA, Salvachúa D, Settle AE, Johnson CW, Menart MJ, Cleveland NS, Ciesielski PN, Steirer KX, Dorgan JR, Beckham GT (2016) cis, cis-Muconic acid: separation and catalysis to bio-adipic acid for nylon-6,6 polymerization. Green Chem 18:3397–3413
Wang H, Ruan H, Feng M, Qin Y, Job H, Luo L, Wang C, Engelhard MH, Kuhn E, Chen X (2017) One-pot process for hydrodeoxygenation of lignin to alkanes using Ru-based bimetallic and bifunctional catalysts supported on zeolite Y. Chemsuschem 10:1846–1856
Wilder HD, Daleski EJ Jr (1965) Part II of a series on kraft pulping kinetics. Tappi 48(5):293–297
Wu X, Fan X, Xie S, Lin J, Cheng J, Zhang Q, Chen L, Wang Y (2018) Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat Catal 1:772–780
Xu W, Miller SJ, Agrawal PK, Jones CW (2012) Depolymerization and hydrodeoxygenation of switchgrass lignin with formic acid. Chemsuschem 5:667–675
Yan N, Zhao C, Dyson PJ, Wang C, Liu LT, Kou Y (2008) Selective degradation of wood lignin over Noble-metal catalysts in a two-step process. Chemsuschem 1:626–629
Yasuda S, Ota K (1987) Chemical structure of sulfuric acid lignin. Pt. XX. Reaction of syringylglycerol-β-syringyl ether and condensation of syringyl nucleus with guaiacyl lignin model compounds in sulfuric acid. Holzforschung 41:59–65
Yu H, Hu J, Fan J, Chang J (2012) One-Pot conversion of sugars and lignin in ionic liquid and recycling of ionic liquid. Ind Eng Chem Res 51:3452–3457
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599
Zhang C, Li H, Lu J, Zhang X, Macarthur KE, Heggen M, Wang F (2017) Promoting lignin depolymerization and restraining the condensation via an oxidation-hydrogenation strategy. ACS Catal 7:3419–3429
Zhao C, Kou Y, Lemonidou AA, Li X, Lercher JA (2010) Hydrodeoxygenation of bio-derived phenols to hydrocarbons using Raney Ni and Nafion/SiO2 catalysts. Chem Commun 46:412–414
Zhao C, He J, Lemonidou AA, Li X, Lercher JA (2011) Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J Catal 280:8–16
Zhou XF (2014) Selective oxidation of kraft lignin over zeolite-encapsulated Co(II) [H4]salen and [H2]salen complexes. J Appl Polym Sci 131:40809–40819
Acknowledgement
This publication has been produced with the financial assistance of Estonia-Russia Cross-Border Cooperation Program 2014–2020, Project ER 30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Evstigneyev, E.I., Shevchenko, S.M. Lignin valorization and cleavage of arylether bonds in chemical processing of wood: a mini-review. Wood Sci Technol 54, 787–820 (2020). https://doi.org/10.1007/s00226-020-01183-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-020-01183-4