Abstract
Purpose
Polycystic ovary syndrome (PCOS), considered a lifelong condition, manifests mainly as a cluster of hyperandrogenic symptoms during the early reproductive years, with the affected woman gradually developing an adverse cardiometabolic profile over the years. However, some data point to the possibility of differences in the evolution of PCOS according to a woman’s weight. The aim of the present study was to evaluate the metabolic and hormonal profiles of women with PCOS over time.
Methods
A total of 763 lean women with PCOS (BMI 20–25 kg/m2) and 376 controls were included. The study group was further divided into three age groups representing women post-adolescence, of reproductive age, and of late reproductive age. All subjects were assessed clinically, biochemically, and hormonally.
Results
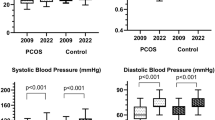
Waist circumference, lipids, androgens, and insulin resistance index (homeostasis model assessment of IR index (HOMA-IR)) were significantly higher in the PCOS group compared with controls. Age subgroup analysis showed a progressive decrease of HOMA-IR and waist circumference, and lipid levels were comparable between PCOS and controls in all age groups. Androgens remained significantly higher in PCOS, but they gradually decreased through time. A significant negative association of age with waist circumference, androgens, insulin, and HOMA-IR was revealed. Univariate and multivariate regression analysis disclosed a strong correlation of HOMA-IR with age (p = 0.014, β − 0.19, SE coefficient 0.008) as a single parameter or in combination with total cholesterol (TC) (p < 0.001, age: β − 0.023, SE 0.10; TC: β 0.084, SE 0.027).
Conclusion
Insulin resistance, androgens, and lipids are gradually improved in an age-dependent manner in lean PCOS women. We hypothesize that if these women do not gain weight with the passage of time, there is a high probability that their cardiometabolic risk will be attenuated.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31:2841–2855. https://doi.org/10.1093/humrep/dew218
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R et al (2014) The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 171:129. https://doi.org/10.1530/EJE-14-0253
Torchen LC (2017) Cardiometabolic risk in PCOS: more than a reproductive disorder. Curr Diab Rep 17:137. https://doi.org/10.1007/s11892-017-0956-2
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS et al (2012) Consensus on womens health aspects of polycystic ovary syndrome (PCOS). Hum Reprod 27:14–24. https://doi.org/10.1016/j.fertnstert.2011.09.024
Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, Norman RJ, Costello MF (2011) Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust 195:S65
Schmidt J, Brännström M, Landin-Wilhelmsen K, Dahlgren E (2011) Reproductive hormone levels and anthropometry in postmenopausal women with polycystic ovary syndrome (PCOS): a 21-year follow-up study of women diagnosed with PCOS around 50 years ago and their age-matched controls. J Clin Endocrinol Metab 96:2178–2185. https://doi.org/10.1210/jc.2010-2959
Welt CK, Carmina E (2013) Lifecycle of polycystic ovary syndrome (PCOS): from in utero to menopause. J Clin Endocrinol Metab 98:4629–4638. https://doi.org/10.1210/jc.2013-2375
Carmina E, Campagna AM, Lobo RA (2013) Emergence of ovulatory cycles with aging in women with polycystic ovary syndrome (PCOS) alters the trajectory of cardiovascular and metabolic risk factors. Hum Reprod 28:2245–2252. https://doi.org/10.1093/humrep/det119
Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, De Jong FH, Fauser BC, Laven JS (2011) The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril 96:1259–1264. https://doi.org/10.1016/j.fertnstert.2011.09.002
Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, Pasquali R (2012) Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes 61:2369–2374. https://doi.org/10.2337/db11-1360
Livadas S (2018) Letter to the editor: “Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome”. J Clin Endocrinol Metab 103:360–361. https://doi.org/10.1210/jc.2017-02051
Livadas S, Kollias A, Panidis D, Diamanti-Kandarakis E (2014) Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: evidence from 1345 women with the syndrome. Eur J Endocrinol 171:301–309. https://doi.org/10.1530/EJE-13-1007
Alvarez-Blasco F, Botella-Carretero JI, San Millán JL, Escobar-Morreale HF (2006) Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med 166:2081–2086
Livadas S, Dracopoulou M, Dastamani A, Sertedaki A, Maniati-Christidi M, Magiakou AM, Kanaka-Gantenbein C, Chrousos GP, Dacou-Voutetakis C (2015) The spectrum of clinical, hormonal and molecular findings in 280 individuals with nonclassical congenital adrenal hyperplasia caused by mutations of the CYP21A2 gene. Clin Endocrinol 82:543–549. https://doi.org/10.1111/cen.12543
Witchel SF, Azziz R (2010) Nonclassic congenital adrenal hyperplasia. Int J Pediatr Endocrinol 625105. https://doi.org/10.1155/2010/625105
David D, Norenberg DD (1984) Lipid research clinics program. JAMA 252:2545
Mathur RS, Moody LO, Landgrebe S, Williamson HO (1981) Plasma androgens and sex hormone-binding globulin in the evaluation of hirsute females. Fertil Steril 35:29–35
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS (1998) Mortality of woman with polycystic ovary syndrome at long term follow up. J Clin Epidemiol 51:581–586
Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH (2000) Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 20:2414–2421
Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF et al (2013) Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 28:777–784. https://doi.org/10.1093/humrep/des463
Kirchengast S, Huber J (2001) Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod 16:1255–1260
Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G (2007) Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 92:2500–2505
Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, Dumesic DA, Azziz R (2013) Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab 98:1541–1548. https://doi.org/10.1210/jc.2012-2937
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33:981–1030. https://doi.org/10.1210/er.2011-1034
Baillargeon J-P, Carpentier A (2007) Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril 88:886–893
Bili H, Laven J, Imani B, Eijkemans MJ, Fauser BC (2001) Age-related differences in features associated with polycystic ovary syndrome in normogonadotrophic oligo-amenorrhoeic infertile women of reproductive years. Eur J Endocr 145:749–755
Davison SL, Bell R, Donath S, Montalto JG, Davis SR (2005) Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 90:3847–3853
Puurunen J, Piltonen T, Jaakkola P, Ruokonen A, Morin-Papunen L, Tapanainen JS (2009) Adrenal androgen production capacity remains high up to menopause in women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1973–1978. https://doi.org/10.1210/jc.2008-2583
Pinola P, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, Stener-Victorin E, Ruokonen A, Puukka K, Tapanainen JS, Morin-Papunen LC (2015) Androgen profile through life in women with polycystic ovary syndrome: a Nordic multicenter collaboration study. J Clin Endocrinol Metab 100:3400–3407. https://doi.org/10.1016/j.fertnstert.2016.12.017
Nestler JE, Strauss JF III (1991) Insulin as an effector of human ovarian and adrenal steroid metabolism. Endocr Metabol Clin 4:807–823
Macut D, Bjekić-Macut J, Savić-Radojević A (2013) Dyslipidemia and oxidative stress in PCOS. Front Horm Res 40:51–63. https://doi.org/10.1159/000341683
Spałkowska M, Mrozińska S, Gałuszka-Bednarczyk A, Gosztyła K, Przywara A, Guzik J, Janeczko M, Milewicz T, Wojas-Pelc A (2018) The PCOS patients differ in lipid profile according to their phenotypes. Exp Clin Endocrinol Diabetes 126:437–444. https://doi.org/10.1055/s-0043-121264
Gourgari E, Lodish M, Shamburek R, Keil M, Wesley R, Walter M, Sampson M, Bernstein S, Khurana D, Lyssikatos C et al (2015) Lipoprotein particles in adolescents and young women with PCOS provide insights into their cardiovascular risk. J Clin Endocrinol Metab 100:4291–4298. https://doi.org/10.1210/jc.2015-2566
Li S, Chu Q, Ma J, Sun Y, Tao T, Huang R, Liao Y, Yue J, Zheng J, Wang L et al (2017) Discovery of novel lipid profiles in PCOS: do insulin and androgen oppositely regulate bioactive lipid production? J Clin Endocrinol Metab 102:810–821. https://doi.org/10.1210/jc.2016-2692
Panidis D, Macut D, Tziomalos K, Papadakis E, Mikhailidis K, Kandaraki EA et al (2013) Prevalence of metabolic syndrome in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 78:586–592. https://doi.org/10.1111/cen.12008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The institutional review board of all participating hospitals approved the study. The institutional review boards that approved this study were the Ethical Committee for Human Studies of the Metropolitan Hospital, of Hellenic Red Cross Hospital and of Faculty of Medicine, University of Belgrade, of Faculty of Medicine, University of Wrocław and of Faculty of Medicine, University of Thessaloniki.
Informed consent
Informed consent was obtained from all women.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Livadas, S., Macut, D., Bothou, C. et al. Insulin resistance, androgens, and lipids are gradually improved in an age-dependent manner in lean women with polycystic ovary syndrome: insights from a large Caucasian cohort. Hormones 19, 531–539 (2020). https://doi.org/10.1007/s42000-020-00211-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-020-00211-z