Abstract
Background
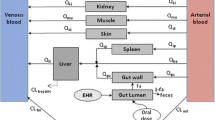
Predicting drug pharmacokinetics in pregnant women including placental drug transfer remains challenging. This study aimed to develop and evaluate maternal–fetal physiologically based pharmacokinetic models for two antiretroviral drugs, dolutegravir and raltegravir.






Similar content being viewed by others
References
Taylor AW, Nesheim SR, Zhang X, Song R, Fitzharris LF, Lampe MA, et al. Estimated perinatal HIV infection among infants born in the United States, 2002–2013. JAMA Pediatr. 2017;171(5):435–42.
Nesheim SR, Fitzharris LF, Lampe MA, Gray KM. Reconsidering the number of women with HIV infection who give birth annually in the United States. Public Health Rep. 2018;133(6):637–43.
Nesheim S, Taylor A, Lampe MA, Kilmarx PH, Fitz Harris L, Whitmore S, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012;130(4):738–44.
Caritis SN, Bastian JR, Zhang H, Kalluri H, English D, England M, et al. An evidence-based recommendation to increase the dosing frequency of buprenorphine during pregnancy. Am J Obstet Gynecol. 2017;217(4):459.e1–6.
Krishna R, East L, Larson P, Valiathan C, Deschamps K, Luk JA, et al. Atazanavir increases the plasma concentrations of 1200 mg raltegravir dose. Biopharm Drug Dispos. 2016;37(9):533–41.
Rahangdale L, Cates J, Potter J, Badell ML, Seidman D, Miller ES, et al. Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. Am J Obstet Gynecol. 2016;214(3):385.e1–7.
World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/. Accessed 18 Nov 2019.
National Institutes of Health. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. 2018. https://aidsinfo.nih.gov/guidelines/html/3/perinatal/0. Accessed 18 Nov 2019.
Dallmann A, Pfister M, Van Den Anker J, Eissing T. Physiologically based pharmacokinetic modeling in pregnancy: a systematic review of published models. Clin Pharmacol Ther. 2018;104(6):1110–24.
Yap CW. PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem. 2011;32(7):1466–74.
Dallmann A, Solodenko J, Ince I, Eissing T. Applied concepts in PBPK modeling: how to extend an open systems pharmacology model to the special population of pregnant women. CPT Pharmacomet Syst Pharmacol. 2018;7(7):419–31.
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–61.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56(11):1303–30.
Dallmann A, Ince I, Coboeken K, Eissing T, Hempel G. A physiologically based pharmacokinetic model for pregnant women to predict the pharmacokinetics of drugs metabolized via several enzymatic pathways. Clin Pharmacokinet. 2018;57(6):749–68.
Mian P, Van Den Anker JN, Van Calsteren K, Annaert P, Tibboel D, Pfister M, et al. Physiologically based pharmacokinetic modeling to characterize acetaminophen pharmacokinetics and N-acetyl-p-benzoquinone imine (NAPQI) formation in non-pregnant and pregnant women. Clin Pharmacokinet. 2020;59(1):97–110. https://doi.org/10.1007/s40262-019-00799-5.
Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review (s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204790Orig1s000ClinPharmR.pdf. Accessed 8 Oct 2019.
Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, et al. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother. 2013;57(8):3536–46.
Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–7.
Song IH, Borland J, Savina PM, Chen S, Patel P, Wajima T, et al. Pharmacokinetics of single-dose dolutegravir in HIV-seronegative subjects with moderate hepatic impairment compared to healthy matched controls. Clin Pharmacol Drug Dev. 2013;2(4):342–8.
Song I, Weller S, Patel J, Borland J, Wynne B, Choukour M, et al. Effect of carbamazepine on dolutegravir pharmacokinetics and dosing recommendation. Eur J Clin Pharmacol. 2016;72(6):665–70.
Song I, Borland J, Chen S, Patel P, Wajima T, Peppercorn A, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56(3):1627–9.
Weller S, Borland J, Chen S, Johnson M, Savina P, Wynne B, et al. Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol. 2014;70(1):29–35.
Ford SL, Gould E, Chen S, Margolis D, Spreen W, Crauwels H, et al. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother. 2013;57(11):5472–7.
Johnson M, Borland J, Chen S, Savina P, Wynne B, Piscitelli S. Effects of boceprevir and telaprevir on the pharmacokinetics of dolutegravir. Br J Clin Pharmacol. 2014;78(5):1043–9.
Wang X, Cerrone M, Ferretti F, Castrillo N, Maartens G, Mcclure M, et al. Pharmacokinetics of dolutegravir 100 mg once daily with rifampicin. Int J Antimicrob Agents. 2019;54(2):202–6.
Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254–8.
Kassahun K, Mcintosh I, Cui D, Hreniuk D, Merschman S, Lasseter K, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos. 2007;35(9):1657–63.
Laufer R, Paz OG, Di Marco A, Bonelli F, Monteagudo E, Summa V, et al. Quantitative prediction of human clearance guiding the development of raltegravir (MK-0518, isentress) and related HIV integrase inhibitors. Drug Metab Dispos. 2009;37(4):873–83.
Mulligan N, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018;32(6):729–37.
Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67(4):375–81.
Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: a randomised trial (DolPHIN-1 study). PLoS Med. 2019;16(9):e1002895.
Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsacker K, Haberl AE, et al. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis. 2015;61(5):809–16.
Dallmann A, Ince I, Solodenko J, Meyer M, Willmann S, Eissing T, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56(12):1525–41.
Zhang Z, Unadkat JD. Development of a novel maternal–fetal physiologically based pharmacokinetic model II: verification of the model for passive placental permeability drugs. Drug Metab Dispos. 2017;45(8):939–46.
Griessinger JA, Hauptstein S, Laffleur F, Netsomboon K, Bernkop-Schnurch A. Evaluation of the impact of multivalent metal ions on the permeation behavior of dolutegravir sodium. Drug Dev Ind Pharm. 2016;42(7):1118–26.
Moss DM, Kwan WS, Liptrott NJ, Smith DL, Siccardi M, Khoo SH, et al. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob Agents Chemother. 2011;55(2):879–87.
Open Systems Pharmacology: Compounds: Definition and Work Flows. https://docs.open-systems-pharmacology.org/working-with-pk-sim/pk-sim-documentation/pk-sim-compounds-definition-and-work-flow#adme-properties. Accessed 29 Feb 2020.
Poulin P, Schoenlein K, Theil FP. Prediction of adipose tissue: plasma partition coefficients for structurally unrelated drugs. J Pharm Sci. 2001;90(4):436–47.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci. 2002;91(1):129–56.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Takaku T, Nagahori H, Sogame Y, Takagi T. Quantitative structure-activity relationship model for the fetal–maternal blood concentration ratio of chemicals in humans. Biol Pharm Bull. 2015;38(6):930–4.
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123(5):369–75.
Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. 1981;196(1):257–60.
Collier AC, Thevenon AD, Goh W, Hiraoka M, Kendal-Wright CE. Placental profiling of UGT1A enzyme expression and activity and interactions with preeclampsia at term. Eur J Drug Metab Pharmacokinet. 2015;40(4):471–80.
Bollen P, Freriksen J, Konopnicki D, Weizsacker K, Hidalgo Tenorio C, Molto J, et al. The effect of pregnancy on the pharmacokinetics of total and unbound dolutegravir and its main metabolite in women living with human immunodeficiency virus. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa006.
Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38(1):62–75.
Dallmann A, Liu XI, Burckart GJ, Van Den Anker J. Drug transporters expressed in the human placenta and models for studying maternal–fetal drug transfer. J Clin Pharmacol. 2019;59(Suppl. 1):S70–81.
Pfrunder A, Gutmann H, Beglinger C, Drewe J. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol. 2003;55(1):59–66.
Nakumura T, Sakaeda T, Ohmoto N, Moriya Y, Komoto C, Shirakawa T, et al. Gene expression profiles of ABC transporters and cytochrome P450 3A in Caco-2 and human colorectal cancer cell lines. Pharm Res. 2003;20(2):324–7.
Zrieki A, Farinotti R, Buyse M. Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm Res. 2008;25(9):1991–2001.
Yano K, Shimizu S, Tomono T, Ogihara T. Gastrointestinal hormone cholecystokinin increases P-glycoprotein membrane localization and transport activity in Caco-2 cells. J Pharm Sci. 2017;106(9):2650–6.
Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27(6–7):602–9.
Wang C, Li H, Luo C, Li Y, Zhang Y, Yun D, et al. The effect of maternal obesity on the expression and functionality of placental P-glycoprotein: implications in the individualized transplacental digoxin treatment for fetal heart failure. Placenta. 2015;36(10):1138–47.
Bataille A, Rousset J, Marret E, Bonnet F. Ultrasonographic evaluation of gastric content during labour under epidural analgesia: a prospective cohort study. Br J Anaesth. 2014;112(4):703–7.
Davison JS, Davison MC, Hay DM. Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw. 1970;77(1):37–41.
Whitehead EM, Smith M, Dean Y, O’sullivan G. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48(1):53–7.
Stillhart C, Vucicevic K, Augustijns P, Basit AW, Batchelor H, Flanagan TR, et al. Impact of gastrointestinal physiology on drug absorption in special populations: an UNGAP review. Eur J Pharm Sci. 2020;147:105280.
De Sousa MM, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81(4):646–57.
Schalkwijk S, Buaben AO, Freriksen JJM, Colbers AP, Burger DM, Greupink R, et al. Prediction of fetal darunavir exposure by integrating human ex-vivo placental transfer and physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2018;57(6):705–16.
Liu XI, Momper JD, Rakhmanina N, Van Den Anker JN, Green DJ, Burckart GJ, et al. Physiologically based pharmacokinetic models to predict maternal pharmacokinetics and fetal exposure to emtricitabine and acyclovir. J Clin Pharmacol. 2020;60(2):240–55.
Schalkwijk S, Greupink R, Colbers AP, Wouterse AC, Verweij VG, Van Drongelen J, et al. Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model. J Antimicrob Chemother. 2016;71(2):480–3.
Hill MD, Abramson FP. The significance of plasma protein binding on the fetal/maternal distribution of drugs at steady-state. Clin Pharmacokinet. 1988;14(3):156–70.
Chignell CF, Vesell ES, Starkweather DK, Berlin CM. The binding of sulfaphenazole to fetal, neonatal, and adult human plasma albumin. Clin Pharmacol Ther. 1971;12(6):897–901.
Krasner J, Giacoia GP, Yaffe SJ. Drug–protein binding in the newborn infant. Ann N Y Acad Sci. 1973;226:101–14.
Herve F, Rajkowski K, Martin MT, Dessen P, Cittanova N. Drug-binding properties of rat alpha 1-foetoprotein: binding of warfarin, phenylbutazone, azapropazone, diazepam, digitoxin and cholic acid. Biochem J. 1984;221(2):401–6.
Hirano K, Watanabe Y, Adachi T, Ito Y, Sugiura M. Drug-binding properties of human alpha-foetoprotein. Biochem J. 1985;231(1):189–91.
US Food and Drug Administration. FDA drug safety communication: FDA to evaluate potenitial risk of neural tube birth defects with HIV medicine dolutegravir (Juluca, Tivicay, Triumeq). 2018. https://www.fda.gov/Drugs/DrugSafety/ucm608112.htm. Accessed 25 Jun 2018.
WHO. Potential safety issue affecting women living with HIV using dolutegravir at the time of conception. 2018. https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf. Accessed 11 Nov 2019.
Zamek-Gliszczynski MJ, Zhang X, Mudunuru J, Du Y, Chen JL, Taskar KS, et al. Clinical extrapolation of the effects of dolutegravir and other HIV integrase inhibitors on folate transport pathways. Drug Metab Dispos. 2019;47(8):890–8.
Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012;56(6):3020–6.
Moss DM, Siccardi M, Back DJ, Owen A. Predicting intestinal absorption of raltegravir using a population-based ADME simulation. J Antimicrob Chemother. 2013;68(7):1627–34.
Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, et al. Minimal effects of ritonavir and efavirenz on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2008;52(12):4338–433.
Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83(2):293–9.
Markowitz M, Morales-Ramirez JO, Nguyen BY, Kovacs CM, Steigbigel RT, Cooper DA, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43(5):509–15.
Rhee EG, Rizk ML, Brainard DM, Gendrano IN 3rd, Jin B, Wenning LA, et al. A pharmacokinetic comparison of adult and paediatric formulations of raltegravir in healthy adults. Antivir Ther. 2014;19(6):619–24.
Wenning LA, Hanley WD, Brainard DM, Petry AS, Ghosh K, Jin B, et al. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2009;53(7):2852–6.
Iwamoto M, Wenning LA, Nguyen BY, Teppler H, Moreau AR, Rhodes RR, et al. Effects of omeprazole on plasma levels of raltegravir. Clin Infect Dis. 2009;48(4):489–92.
Brainard DM, Friedman EJ, Jin B, Breidinger SA, Tillan MD, Wenning LA, et al. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J Clin Pharmacol. 2011;51(3):422–7.
Taburet AM, Sauvageon H, Grinsztejn B, Assuied A, Veloso V, Pilotto JH, et al. Pharmacokinetics of raltegravir in HIV-infected patients on rifampicin-based antitubercular therapy. Clin Infect Dis. 2015;61(8):1328–35.
Acknowledgements
The authors would like to thank Dr. Ibrahim Ince (Bayer AG) for providing the non-pregnant PBPK model for raltegravir via upload on OSP GitHub. The opinions expressed in this article are those of the authors and should not be interpreted as the position of the US Food and Drug Administration (FDA) or of the National Institutes of Health. No broader FDA policies or perspectives are intended nor should be inferred. The FDA does not recommend any specific PBPK software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xiaomei I. Liu, Jeremiah D. Momper, Natella Y. Rakhmanina, Dionna J. Green, Gilbert J. Burckart, Tim R. Cressey, Mark Mirochnick, Brookie M. Best, John N. van den Anker, and André Dallmann have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. André Dallmann is an employee of Bayer AG and involved in OSP software development. The results from this study were presented in part at the American College of Clinical Pharmacology Annual Meeting, Washington, DC, September 2018.
Funding
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The NIH awards numbers 5T32HD087969-03 and 5T32HD087969-02 also support this project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X.I., Momper, J.D., Rakhmanina, N.Y. et al. Prediction of Maternal and Fetal Pharmacokinetics of Dolutegravir and Raltegravir Using Physiologically Based Pharmacokinetic Modeling. Clin Pharmacokinet 59, 1433–1450 (2020). https://doi.org/10.1007/s40262-020-00897-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00897-9